Extraction of Ce(IV) from sulphuric acid solution by emulsion liquid membrane using D2EHPA as carrier
Jingui Hea,
Yong Lia,
Xiangxin Xue*a,
Hongqiang Rua,
Xiaowei Huangb and
He Yanga
aSchool of Material and Metallurgy, Northeastern University, Shenyang, Liaoning 110819, China. E-mail: xuexx@mail.neu.edu.cn; Xue_Xiangxin@126.com
bNational Engineering Research Center for Rare Earth Materials, General Research Institute for Nonferrous Metals, Grirem Advanced Materials Co., Ltd., Beijing 100088, China
First published on 18th August 2015
Abstract
In order to provide a potential method for extracting Ce(IV), the extraction of Ce(IV) from sulphuric acid solution by an emulsion liquid membrane using D2EHPA as a carrier was investigated. The ELM system consisted of sulfonated kerosene as diluent, Span 80 as surfactant, liquid paraffin as intensifier and hydrochloric acid containing hydrogen peroxide as the inner aqueous solution. The influences of various parameters on the extraction of Ce(IV) were investigated. The optimum conditions for Ce(IV) extraction can be summarized as follows: D2EHPA concentration, 12% (v/v); Span 80 concentration, 2–3% (v/v); liquid paraffin concentration, 2–4% (v/v); hydrochloric acid concentration in the internal phase, 4–5 mol l−1; hydrogen peroxide concentration, 0.02 mol l−1; volume ratio of membrane phase to internal phase (Roi), 1.5; external phase acidity, 0.4–0.5 mol l−1; volume ratio of external phase to membrane phase (Rwe), 2; extraction time, 15 min; and stirring speed, 250 rpm. Experiments in which Ce(IV) was separated from RE(III) were then carried out under the optimum conditions, and the results indicated that the system is extremely selective for Ce(IV). The mechanism of Ce(IV) extraction has also been discussed. The loss for the experimental process was within 3%. The results reveal that the ELM method is a clean and cost-effective process for the extraction of Ce(IV) from sulphuric acid solution.
1 Introduction
Cerium, one of most abundant of the light rare earth elements, is widely used in industry, mainly in the form of ceria (CeO2), as a material for ceramics, catalysts, polishing powders and so on.1,2 Solvent extraction is an important method for the separation and purification of cerium and is widely used in industrial production because of its simplicity, speed, and applicability in the extraction and separation of rare earth elements.3,4 The most commonly used extractants are organophosphorus acids that belong to a compound formation class, such as di-(2-ethylhexyl) phosphoric acid (D2EHPA) and 2-ethylhexyl phosphonic acid-2-ethylhexyl ester (HEHEHP).5 The conventional solvent extraction method possesses many deficiencies, such as complex multi-stage extraction operation, larger volumes of solvent and associated equipment, high energy consumption, and higher investment costs.6 Therefore, the development of more efficient extraction techniques has attracted much attention and has important significance in the development of the rare earth industry. Liquid membrane technology is gaining importance and is emerging as an attractive and promising technology for the concentration and separation of metal ions from aqueous solutions.7There are several different types of liquid membranes, such as bulk (BLM), supported (SLM), and emulsion (ELM) liquid membranes, polymer inclusion membranes (PIMs) and hollow fiber liquid membranes (HFSLMs).8,9 BLMs consist of an aqueous feed and stripping phase separated by an immiscible membrane phase. BLMs are often used to study the transport properties of novel carriers; however, their small membrane surface areas make them unattractive.10 SLMs have essentially the same configuration as BLMs, except that the organic phase is contained in the pores of a macroporous polymer sheet.9 SLMs are very useful for laboratory research and development purposes, but the surface area to volume ratio of flat sheets is too low for industrial applications.11 Among SLMs, HFSLM is the most promising. It possesses several advantages such as simultaneous one-step extraction and stripping, high selectivity, large surface area, high mass transfer rate, and low extractant and energy consumption.12 Despite its advantages, the large-scale application of HFSLM is still very limited due to its insufficient membrane stability.13 In addition, its surface active effects can lead to fouling, and the variable geometry makes it difficult to model.7 The PIMs, which are composed of polymers, plasticizers, and ion carriers, have gained considerable attention due to their higher selectivities for target metal ions, lower carrier consumption, and the integrated extraction and stripping process; however, the selection of a suitable polymer for PIMs is one of the important factors in membrane separation.14
Emulsion liquid membrane (ELM), a separation technique proposed by Li,15 has been regarded as an emerging energy-efficient technology that combines the characteristics of solid membrane separation and solvent extraction. The main advantages of ELM are as follows: high interfacial area for mass transfer because of the small sizes of the aqueous droplets; high diffusion rate of the target ion through the membrane; simultaneous, one-step extraction and stripping; the capability of treating a variety of elements and compounds in the industrial setting at a greater speed and with a high degree of effectiveness, with varying contaminant concentrations and volume requirements.16 Compared with HFSLM, ELM requires a much smaller inventory of solvent and carrier since the volume ratios of the two aqueous phases to the membrane are large; however, the large volume ratios are a disadvantage in that very hydrophobic membrane solvents and carriers are required to maintain membrane integrity and operate the system.7
The ELM contains three phases, the membrane phase, internal phase and external phase. The liquid membrane consists of a diluent, a surfactant and a carrier reagent (extractant) to separate the solute by chemical reaction. The external continuous phase and internal phase are separated by the liquid membrane. The selective component is transported through the liquid membrane from the external phase and concentrated in the internal phase.17 After extraction, the loaded emulsion is separated from the feed solution, and demulsification yields a membrane phase that can be recycled.6 The extraction mechanism involved in liquid membrane transport is essentially the same as that in solvent extraction. It has been reported that various ions can be successfully extracted by ELM.18–23 Few papers have been reported on the extraction of lanthanides by ELM.24–26 Teramoto et al.27 and Tang and Wai28 explored the extraction and transport of trivalent lanthanides by liquid membrane systems.
The transport of Ce(IV) with a membrane system has been reported before;29–31 however, studies on Ce(IV) extraction from sulphuric acid solution by ELM are lacking.
In this paper, the extraction of Ce(IV) from sulphuric acid solution by ELM was investigated. D2EHPA was chosen as the extractant. Various parameters influencing the extraction of Ce(IV) were investigated with the aim of providing potential applications for extracting Ce(IV) by a clean and cost-effective separation method, replacing the existing system.
2 Experimental
2.1 Reagents and apparatus
Liquid paraffin with a specific gravity of 0.835–0.890 was supplied by Tianjin Bodi Chemical Industry Co., Ltd. Span80 (C24H44O6), hydrogen peroxide (H2O2) and cerium(IV) sulphate (Ce(SO4)2·4H2O) were of analytical grade and supplied by Shenyang Guoyao Group Chemical Reagent Co., Ltd. Sulphuric acid (A.R. 98%) and hydrochloric acid (A.R. 37.5%) were supplied by Shenyang Lab Science and Trade Co., Ltd. n-Dodecane, D2EHPA and sulfonated kerosene were supplied by Shanghai Laiyashi Chemical Industry Co., Ltd. All solutions were prepared using deionised water with resistivities >18.23 MΩ cm−1.An FJ200-SH digital high-speed dispersion homogenizer (Shanghai Specimen Model Factory) was used to prepare the liquid membrane solution. A DW-3 speed control magnetic stirrer (Gongyi Yuhua Instruments Co., Ltd) was used to carry out the extraction experiments.
2.2 Procedure
To determine the important variables governing the extraction of Ce(IV), the extractant concentration, surfactant concentration, intensifier concentration, hydrochloric acid and hydrogen peroxide concentrations of the stripping solution, volume ratio of the membrane phase to the internal phase (Roi), volume ratio of the external phase to the membrane phase (Rwe), acidity in the feed solution, extraction time and stirring speed were varied to observe their effects on Ce(IV) extraction. The separation of Ce(IV) from RE(III) was also investigated.
2.3 Analyses methods
The chemical structure of the D2EHPA complex with Ce(IV) was detected by the Nicolet-380 Fourier transform infrared spectrometer (Thermo Electron Corporation). The viscosities of the emulsions were measured by DV2TLVTJ0 viscometer (Brookfield).The average sizes of emulsions were measured using a BT-9300H laser particle size analyser (Dandong Bettersize Instruments Co., Ltd) within a few minutes after preparation. The globule size of the emulsion was measured by diluting the sample with deionised water, and the internal droplet size of the emulsion was measured by diluting the sample with n-dodecane. The average diameters of the emulsion liquid globules and droplets were represented by Sauter mean diameter (d32 = ∑nidi3/∑nidi2, representing a surface average value). Additionally, a BT-1600 particle image analysis system with an optical microscope (Dandong Bettersize Instruments Co., Ltd) was used to view emulsion morphology and assess the average thickness of the membrane.
The concentration of D2EHPA was determined by titration with standard NaOH solution using phenolphthalein and bromophenol blue as indicators. The acidity was determined by neutralisation titration with standard NaOH solution using mixed indicators of methyl red and bromocresol green after masking the metal ions with Ca-EDTA solution. The concentration of RE(III) was determined by titration with standard EDTA solution with xylenol orange as the indicator. The concentration of Ce(IV) was determined by titration with standard [(NH4)2Fe(SO4)2] solution using sodium diphenylamine sulfonate as indicator.
The extraction efficiency η was obtained by eqn (1):
| η = (C0 − Ct)/C0 × 100% | (1) |
3 Results and discussion
3.1 Extraction mechanism in ELM system
D2EHPA is supposed to be present as a dimeric species such as H2A2 in the non-polar diluent sulfonated kerosene due to the presence of intermolecular hydrogen bonds;32 the chemical structure of D2EHPA is shown in Fig. 1. Solvent extraction studies on rare earth elements using D2EHPA suggested that Ce(IV) can be extracted from an aqueous acid medium with D2EHPA via a cation-exchange mechanism.33 The FTIR spectra of D2EHPA complexes with Ce(IV) are presented in Fig. 2. The adsorption peaks at 2316 cm−1, 1685 cm−1, 1233 cm−1 and 1035 cm−1 are attributed to the stretching modes of P–OH bonds, the dimeric peak of hydrogen bonds, the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds and the stretching modes of P–O–C bonds, respectively.34 The band at 2316 cm−1 nearly disappears after extraction, demonstrating the extraction mechanism involving cation-exchange in which Ce(IV) replaces the hydrogen atom in P–OH bonds. The band at 1685 cm−1 also disappears, indicating that the intermolecular hydrogen bonds in D2EHPA are eliminated. The shift of the P
O bonds and the stretching modes of P–O–C bonds, respectively.34 The band at 2316 cm−1 nearly disappears after extraction, demonstrating the extraction mechanism involving cation-exchange in which Ce(IV) replaces the hydrogen atom in P–OH bonds. The band at 1685 cm−1 also disappears, indicating that the intermolecular hydrogen bonds in D2EHPA are eliminated. The shift of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration from 1233 cm−1 to about 1283 cm−1 in the Ce(IV)-loaded D2EHPA reveals the strong interaction between Ce(IV) and P
O stretching vibration from 1233 cm−1 to about 1283 cm−1 in the Ce(IV)-loaded D2EHPA reveals the strong interaction between Ce(IV) and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The peak at 1035 cm−1 has no obvious variation, indicating that there is no chemical bond between Ce(IV) and P–O–C. The extraction reaction between Ce(IV) and D2EHPA in the ELM system is the same as the solvent extraction. According to the results, the saturated extraction mechanism of Ce(IV) by ELM using D2EHPA as carrier in sulphuric acid medium (pH < 1) can be expected to follow eqn (2):3
O. The peak at 1035 cm−1 has no obvious variation, indicating that there is no chemical bond between Ce(IV) and P–O–C. The extraction reaction between Ce(IV) and D2EHPA in the ELM system is the same as the solvent extraction. According to the results, the saturated extraction mechanism of Ce(IV) by ELM using D2EHPA as carrier in sulphuric acid medium (pH < 1) can be expected to follow eqn (2):3| nCe(IV)(a) + 2nH2A2(o) ⇆ (CeA4)n(o) + 4nH(I)(a) | (2) |
 | ||
| Fig. 2 FTIR spectra of (a) sulfonated kerosene, (b) sulfonated kerosene + 12%D2EHPA (v/v) and (c) sulfonated kerosene + 12%D2EHPA (v/v) + Ce(IV). | ||
Hydrogen peroxide can be used as an oxidant as well as a reductant. The standard electrode potentials in hydrochloric acid conditions are as follows:35,36
 | (3) |
From eqn (3), the oxidizing ability of Ce(IV) in acid solution is stronger than that of hydrogen peroxide, so Ce(IV) can oxidize hydrogen peroxide. It can be concluded that using hydrogen peroxide as a reductant to reduce Ce(IV) is thermodynamically feasible and will not bring any impurity ions into the solution. Therefore, hydrochloric acid containing a certain amount of hydrogen peroxide was employed as the internal aqueous phase. The hydrogen peroxide first reduces Ce(IV) to Ce(III), and Ce(III) is then stripped with hydrochloric acid following the equilibrium equation shown as eqn (4):
| 2(CeA4)n(o) + 6nHCl(a) + 3nH2O2(a) ⇆ 2nCeCl3(a) + 4nH2A2(o) + 2nH2O + 2nO2↑ | (4) |
It is concluded that both the equilibrium reactions take place in the ELM system in the following sequence (Fig. 3):
(a) Transport of Ce(IV) ions from the bulk of the external phase to interface1 via the D2EHPA-based membrane.
(b) Formation of the extracted complex at the external phase-liquid membrane interface.
(c) Diffusion of the extracted complex from the membrane phase to interface2.
(d) Stripping of the metal ion from the extracted complex by the internal phase stripping solution (HCl–H2O2) and enrichment of the Ce(III) ions in the internal phase droplets.
As discussed above, the extraction of Ce(IV) by ELM is a reverse-transfer process in which the Ce(IV) and H(I) are transferred from the high-concentration region to the low-concentration region. The Ce(IV) is transferred from the external phase to the internal phase, while the H(I) is transferred from the internal phase to the external phase.
3.2 Emulsion characterisation
Liquid membranes were prepared by emulsifying an aqueous solution of stripping phase (HCl–H2O2) with an organic phase (D2EHPA, Span80 and liquid paraffin in sulfonated kerosene), as discussed in Section 2. Studies carried out with varying emulsifier speed (1000–7000 rpm) indicated that a stable emulsion was obtained only when the emulsifier speed was higher than 2000 rpm. Therefore, an emulsifier speed of 5000 rpm was selected for the preparation of emulsion in all subsequent studies. The ELM was allowed to stand and kept under observation for any physical changes to determine the stability of ELM with respect to time. Photographs of the emulsion at different times are presented in Fig. 4. The ELM was found to be a milky emulsion that is stable for up to 2 h. The emulsion becomes unstable 6 h later and separates into two phases (organic and strip phases) after 12 h.For further observation of the emulsion, a typical photograph of the emulsion is presented in Fig. 5. As shown, the clear and stable W1/O type globules distribute uniformly in the emulsion, and the Sauter mean diameter of the globules is 47.26 μm. There are many droplets in the globules with an average diameter of 1.20 μm. The average thickness is estimated to be approximately 5 μm. Table 1 shows the effects of different components of D2EHPA, Span 80 and liquid paraffin on the viscosity. The table shows that increasing various components concentrations results in increased emulsion viscosity. Based on these results, the prepared emulsion can be applied for all subsequent experiments in the present study, including the extraction of Ce(IV) under various experimental conditions.
| D2EHPA (%v/v) | Viscosity (cp) | Span 80 (%v/v) | Viscosity (cp) | Liquid paraffin (%v/v) | Viscosity (cp) |
|---|---|---|---|---|---|
| 8 | 20.40 | 1 | 12.00 | 0 | 24.00 |
| 10 | 23.70 | 2 | 24.60 | 2 | 28.50 |
| 12 | 24.60 | 3 | 33.10 | 3 | 29.70 |
| 14 | 27.90 | 4 | 45.32 | 4 | 31.32 |
| 16 | 31.20 | 8 | 86.70 | 6 | 33.90 |
3.3 Effect of emulsion composition variables
The higher carrier concentration at the interface between the external phase and the emulsion promotes the transport of solute and the complex of Ce(IV) and D2EHPA. Increasing the amount of carrier to 12% (v/v) results in a Ce(IV) extraction efficiency of over 95%. Upon further increases in carrier concentration, the extraction efficiency shows no obvious change, which may be attributed to an increase in the viscosity, leading to larger globules.38 The literature shows that increases in emulsion viscosity with increasing carrier concentration will limit the extraction rate by affecting the dispersion behaviour of the emulsion.20 Chiha et al.39 reported that increasing the carrier concentration decreases the stability of the emulsion because the larger globules can aggregate quickly. In addition, the increase in the carrier concentration will promote permeation swelling, diluting the stripping solution. The swelling effect is more serious when the D2EHPA concentration is increased.40 A higher concentration of carrier will also increase the cost. Therefore, the optimal carrier concentration is found to be 12% (v/v).
 | ||
| Fig. 8 Photographs of emulsions with different liquid paraffin contents: (a) 2% (v/v) and (b) 6% (v/v). | ||
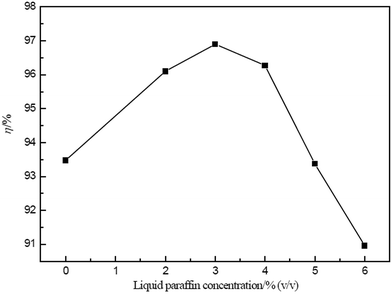
The effect of the liquid paraffin concentration on the extraction of Ce(IV) by ELM is shown in Fig. 9. An extraction efficiency higher than 96% is obtained in the range of 2–4% (v/v), which is considered to be the optimum concentration.
It can be seen that the extraction efficiency is extremely low when hydrogen peroxide is absent in the stripping solution, and the extraction efficiency increases with increasing hydrogen peroxide concentration. When the hydrogen peroxide concentration exceeds 0.02 mol l−1, the extraction efficiency increases slowly. The addition of hydrogen peroxide should be carefully controlled; when the amount added is too little, Ce(IV) cannot be completely reduced and stripped and accumulates in the organic phase, decreasing the extraction ability. However, excess hydrogen peroxide leads to the entrainment of redundant hydrogen peroxide in the organic phase, affecting the extraction efficiency. Thus, a hydrogen peroxide concentration of 0.02 mol l−1 was selected as the most appropriate value.
3.4 Effect of extraction process variables
 | (5) |
 | (6) |
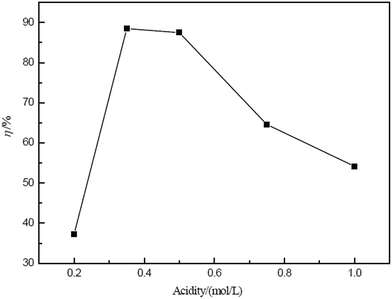
It is evident from eqn (6) that the Ce(IV) extraction efficiency is affected by the volume ratio of the external phase to the membrane phase and by the emulsion droplet diameter.46 An increase in Rwe corresponds to a decrease of the amount of emulsion in the external phase, leading to decreases in the amount of available globules and interfacial surface area per unit volume of the external phase. In addition, the transfer efficiency of Ce(IV) is weakened. Considering the cost, the volume ratio of external phase to membrane phase (Rwe) of 2 was selected as the optimal value.
3.5 The separation of Ce(IV)/RE(III)
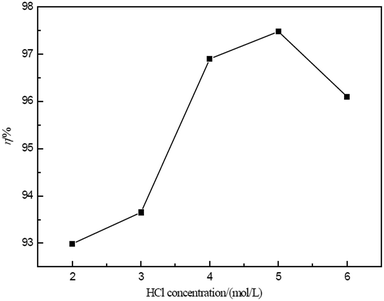
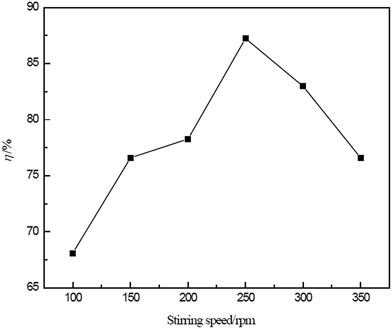
The parameters that affect the extraction of the ELM process were experimentally investigated. The optimum conditions for Ce(IV) extraction can be summarized as follows: D2EHPA concentration, 12% (v/v); Span80 concentration, 2–3% (v/v); liquid paraffin concentration, 2–4% (v/v); hydrochloric acid concentration in the internal phase, 4–5 mol l−1; hydrogen peroxide concentration, 0.02 mol l−1; volume ratio of membrane phase to internal phase (Roi), 1.5; acidity in external phase, 0.4–0.5 mol l−1; volume ratio of external phase to membrane phase (Rwe), 2; extraction time, 15 min; and stirring speed, 250 rpm. The extraction of Ce(IV) was conducted under the experimentally determined optimal conditions, and the obtained extraction efficiency of Ce(IV) was over 98%.RE(III) elements such as La(III), Pr(III), Nd(III) and Sm(III) often accompany Ce(IV) in solution. Under the determined optimum conditions, the separation of Ce(IV) from other RE(III) was investigated.
The separation factor βCe/RE is calculated as follows:
 | (7) |
| Initial concentration in the feed solution (mol l−1) | Extraction efficiency (%) | βCe/RE | |
|---|---|---|---|
| Ce(IV) | RE(III) | ||
| Ce 0.01 + La 0.01 | 98.25 | 0.56 | 175.45 |
| Ce 0.01 + Pr 0.01 | 97.78 | 0.89 | 109.87 |
| Ce 0.01 + Nd 0.01 | 98.54 | 0.75 | 131.39 |
| Ce 0.01 + Sm 0.01 | 98.80 | 0.45 | 219.56 |
At present, many membrane separations for metals have been reported. Table 3 shows some previous studies on metal separation using different emulsion compositions. As can be seen, Span 80 and kerosene are the most widely used surfactant and diluent, respectively. The choices of extractant and internal phase depend on the types of metals being extracted. For rare earth metals, sulphuric acid is most commonly used as the internal phase.
| Solute | Surfactant | Extractant | Internal phase | Diluent | Reference |
|---|---|---|---|---|---|
| Nickel and cobalt | ECA 4360J | 8-HQ | EDTA | Kerosene | 6 |
| Chromium, cobalt, nickel, copper and zinc | Span 80 | TBP | (NH4)2CO3 | Kerosene | 48 |
| Cadmium, zinc, cobalt and nickel | Span 80 | Triotylamine | NH3 | Kerosene | 16 |
| Na2CO3 | |||||
| (NH4)2CO3 | |||||
| CHsCOONH4 | |||||
| Rare earth metals | 2C18Δ9GE | tOct[4]CH2COOH | H2SO4 | Toluene | 50 |
| Rare earth metals | Span 80, 2C18Δ9GEC2QA | PC88A | H2SO4 | n-Heptane | 51 |
| Rare earth metals | 2C18Δ9GE | PC88A | H2SO4 | n-Heptane | 52 |
| 2C18Δ9GEC2QA | |||||
| Rare earth metals | 2C18Δ9GE | tOct[1]CH2COOH | H2SO4 | Toluene | 53 |
| tOct[4]CH2COOH | |||||
| tOct[6]CH2COOH | |||||
| Lead and cadmium | ECA5025 | D2EHPA | HCl, H2SO4 | Tetradecane | 54 |
| Zinc and copper | Span80 | D2EHPA | H2SO4 | Iso-dodecane | 55 |
| Cobalt and nickel | Span80 | TOPO | NH3 | Kerosene | 56 |
| Cobalt and nickel | ECA4360J | Alamine300 | NH4OH | Kerosene | 57 |
| Platinum and palladium | Span80 | TLA,TOMAC,TBP,TOPO,TIBPS | HCl | n-Heptane | 58 |
| PX100 | |||||
| Trace elements: Cd, Co, Cu, Fe, Mn, Ni, Pb, Zn | Span80 | D2EHPA,PC88A | HCl + H2SO4 | Kerosene | 59 |
3.6 Demulsification
The Ce(IV)-loaded emulsion obtained after extraction and separation must be demulsified to recover the metal ions and quantify the extraction efficiency.The stripping efficiency is calculated by eqn (8):
 | (8) |
Five experimental tests were carried out, and the results are illustrated in Fig. 17. A satisfactory mass balance was obtained, and the loss between the external phase and the recovery from the internal phase was within 3%.
 | ||
| Fig. 17 The stripping efficiency after demulsification (experimental conditions: optimum conditions). | ||
4 Conclusions
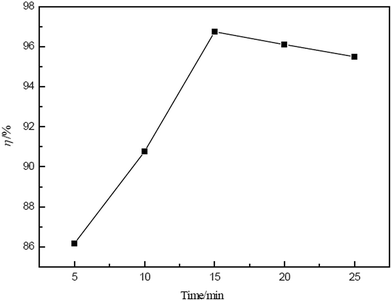
In this study, the extraction of Ce(IV) from sulphuric acid solution by a emulsion liquid membrane comprised of D2EHPA dissolved in sulfonated kerosene as carrier containing Span80 as the emulsifier was investigated. Hydrochloric acid containing hydrogen peroxide was used as the stripping solution. The obtained results can be summarised as follows:1. Stable W1/O-type liquid membranes were prepared at an emulsifier speed of 5000 rpm for 20 min and could be applied for all subsequent experiments.
2. The optimum conditions for Ce(IV) extraction can be summarised as follows: D2EHPA concentration, 12% (v/v); Span80 concentration, 2–3% (v/v); liquid paraffin concentration, 2–4% (v/v); hydrochloric acid concentration in the internal phase, 4–5 mol l−1; hydrogen peroxide concentration, 0.02 mol l−1; volume ratio of membrane phase to internal phase (Roi), 1.5; acidity in external phase, 0.4–0.5 mol l−1; volume ratio of external phase to membrane phase (Rwe), 2; extraction time, 15 min; and stirring speed, 250 rpm. The results demonstrate that among the studied parameters, the D2EHPA concentration, hydrogen peroxide concentration, acidity in the external phase, Rwe and stirring speed play vital roles in Ce(IV) extraction.
3. Under the optimum operating parameters, the extraction efficiency of Ce(IV) is over 98%. The separation of Ce(IV) from RE(III) is realised, showing that the system is extremely selective for Ce(IV).
4. The results obtained demonstrate the validity of the ELM method, which represents an interesting advanced process for the extraction of Ce(IV) from sulphuric acid solution.
Acknowledgements
The financial aid from the following programs are gratefully acknowledged: the key program of National Natural Science Foundation of China (NSFC: 50934004), National Natural Science Foundation of China (51274061), Major State Basic Research Development Program of China (973 Program: 2012CBA01205), and Fundamental Research Supporting Project of Northeastern University (N110602006).References
- X. L. Ren, Q. F. Wei, S. R. Hu and S. J. Wei, Hydrometallurgy, 2010, 103, 205–210 CrossRef CAS PubMed
.
- L. Jelinek, Y. Z. Wei and K. Mikio, J. Rare Earths, 2006, 24, 257–263 CrossRef
.
- W. Y. Wu, Rare Earth Metallurgy, Chemical Industry Press, Beijing, 2005, pp. 71, 112 and 163, in chinese Search PubMed
.
- D. B. Wu, Q. Zhang and B. Borong, Hydrometallurgy, 2007, 88, 210–215 CrossRef CAS PubMed
.
- S. H. Yin, W. Y. Wu, X. Bian and F. Y. Zhang, Hydrometallurgy, 2013, 131–132, 133–137 CrossRef CAS PubMed
.
- R. A. Kumbasar and S. Kasap, Hydrometallurgy, 2009, 95, 121–126 CrossRef CAS PubMed
.
- R. M. Izatt, J. Inclusion Phenom. Macrocyclic Chem., 1997, 29, 197–220 CrossRef CAS
.
- L. Brinchi, R. Germani, M. V. Mancini, G. Savelli and N. Spreti, Eur. J. Org. Chem., 2004, 1330–1335 CrossRef CAS PubMed
.
- H. C. Visser, D. N. Reinhoudt and F. de Jong, Chem. Soc. Rev., 1994, 75–81 RSC
.
- N. M. Kocherginsky, Q. Yang and L. Seelam, Sep. Purif. Technol., 2007, 53, 171–177 CrossRef CAS PubMed
.
- R. A. Bartsch and J. D. Way, ACS Symp. Ser., 2009, 642, 1–10 CrossRef PubMed
.
- P. Ramakul, U. Mooncluen, Y. Yanachawakul and N. Leepipatpiboon, J. Ind. Eng. Chem., 2012, 18, 1606–1611 CrossRef CAS PubMed
.
- A. M. Neplenbroek, D. Bargeman and C. A. Smolders, J. Membr. Sci., 1992, 67, 121–132 CrossRef CAS
.
- L. Guo, Y. H. Liu, C. Zhang and J. Chen, J. Membr. Sci., 2011, 372, 314–321 CrossRef CAS PubMed
.
- N. N. Li, Separating hydrocarbons with liquid membranes, US Pat., 3, 410, 794, 1968
.
- R. A. Kumbasar, Hydrometallurgy, 2009, 95, 290–296 CrossRef CAS PubMed
.
- M. T. A. Reis and J. M. R. Carvalho, J. Membr. Sci., 2004, 237, 97–107 CrossRef CAS PubMed
.
- A. B. Lende, M. K. Dinker, V. K. Bhosale, S. P. Kamble, P. D. Meshramc and P. S. Kulkarni, RSC Adv., 2014, 4, 52316–52323 RSC
.
- Z. F. Guo, H. D. Su, R. Cai and X. L. Ma, RSC Adv., 2015, 7, 1860–1865 CAS
.
- B. Sengupta, R. Sengupta and N. Subrahmanyam, Hydrometallurgy, 2006, 84(1–2), 43–53 CrossRef CAS PubMed
.
- J. Fang, M. Li and Z. Xu, Sep. Sci. Technol., 2003, 38, 3553–3574 CrossRef CAS PubMed
.
- N. Othman, H. Mat and M. Goto, J. Membr. Sci., 2006, 282, 171–177 CrossRef CAS PubMed
.
- D. He and M. Ma, Hydrometallurgy, 2000, 56, 157–170 CrossRef CAS
.
- T. Oshima, T. Kakoi, F. Kubota, K. Ohto and M. Goto, Sep. Sci. Technol., 1998, 33, 1905–1917 CrossRef CAS PubMed
.
- Z. N. Xie, Q. L. Chen and L. J. Zhao, Chin. J. Process Eng., 2013, 13, 197–201 CAS
, in chinese.
- R. H. Zhang and L. Xiao, J. Chin. Rare Earth Soc., 1984, 2, 30–34 CAS
.
- M. Teramoto, T. Sakuramoto, T. Koyama, H. Matsuyama and Y. Miyake, Sep. Sci. Technol., 1986, 21, 229–250 CrossRef CAS PubMed
.
- J. Tang and C. Wai, J. Membr. Sci., 1988, 35, 339–345 CrossRef CAS
.
- A. M. Chaudry, A. Shahid and M. M. Tayyib, Sep. Sci. Technol., 1996, 31, 1309–1326 CrossRef PubMed
.
- R. Prakorn, P. Weerawat and P. Ura, Korean J. Chem. Eng., 2006, 23, 85–92 CrossRef CAS
.
- L. Pei, L. M. Wang and Z. Y. Ma, Front. Environ. Sci. Eng., 2014, 8, 503–509 CrossRef CAS
.
- G. X. Xu and C. Y. Yuan, Solvent extraction of rare earths, Science Press, Beijing, 1987, p. 127 and 128, in chinese Search PubMed
.
- D. Q. Li, Z. H. Wang, G. F. Zeng and Z. Y. Xue, J. Chin. Rare Earth Soc., 1984, 2, 9–19 CAS
.
- X. Q. Sun, Y. Ji, F. C. Hu, B. He, J. Chen and D. Q. Li, Talanta, 2010, 81, 1877–1883 CrossRef CAS PubMed
.
- X. L. Lv, Inorganic peroxide chemical, Science and Technology Press, Beijing, 1987, in chinese Search PubMed
.
- E. wadsorth, F. K. Dukeand and C. A. Goetz, Anal. Chem., 1957, 29, 1824 CrossRef
.
- B. Sengupta, M. S. Bhakhar and R. Sengupta, Hydrometallurgy, 2007, 89, 311–318 CrossRef CAS PubMed
.
- S. Chaouchi and O. Hamdaoui, Sep. Purif. Technol., 2014, 129, 32–40 CrossRef CAS PubMed
.
- M. Chiha, M. H. Samar and O. Hamdaoui, Desalination, 2006, 194, 69–80 CrossRef CAS PubMed
.
- R. S. Juang and K. H. Lin, Colloids Surf., A, 2004, 238, 43–49 CrossRef CAS PubMed
.
- S. H. Lin, C. L. Pan and H. G. Leu, Chem. Eng. J., 2002, 87, 163–169 CrossRef CAS
.
- H. R. Mortaheb, M. H. Amini, F. Sadeghian, B. Mokhtarani and H. Daneshyar, J. Hazard. Mater., 2008, 160, 582–588 CrossRef CAS PubMed
.
- L. Yang, Z. Zhang, Y. Guo, X. Gao and H. Takeuchi, Sep. Purif. Technol., 2005, 47, 88–94 CrossRef CAS PubMed
.
- R. S. Juang and K. H. Lin, Colloids Surf., A, 2004, 238, 43–49 CrossRef CAS PubMed
.
- R. Sabry, A. Hafez, M. Khedr and A. El-Hassanin, Desalination, 2007, 212, 165–175 CrossRef CAS PubMed
.
- R. A. Kumbasar and O. Tutkun, Hydrometallurgy, 2004, 75, 111–121 CrossRef CAS
.
- K. Kondo, K. Kita, I. Koida, J. Irie and F. Nakashio, J. Chem. Eng. Jpn., 1979, 12, 203–209 CrossRef CAS
.
- R. A. Kumbasar, Hydrometallurgy, 2010, 178, 875–882 CAS
.
- P. S. Kulkarni and V. V. Mahajan, J. Membr. Sci., 2012, 201, 123–135 CrossRef
.
- T. Kakoi, T. Oshima, T. Nishiyori, F. Kubota, M. Goto, S. Shinkai and F. Nakashio, J. Membr. Sci., 1998, 143, 125–135 CrossRef CAS
.
- K. Uezu, S. Irie, O. Yoshimura, M. Goto and F. Nakashio, Trans. IchemE, 1997, 75, 513–518 CrossRef CAS
.
- M. Goto, T. Kakoi, N. Yoshii, K. Kondo and F. Nakashio, Ind. Eng. Chem. Res., 1993, 32, 1681–1685 CrossRef CAS
.
- T. Kakoi, T. Nishiyori, T. Oshima, F. Kubota, M. Goto, S. Shinkai and F. Nakashio, J. Membr. Sci., 1997, 136, 261–271 CrossRef CAS
.
- B. J. Raghuraman, N. P. Tirmizi, B. S. Kim and J. M. Wiencek, Environ. Sci. Technol., 1995, 29, 979–984 CrossRef CAS
.
- E. A. Fouad, Chem. Eng. Technol., 2008, 31, 370–376 CrossRef CAS
.
- R. A. Kumbasar, Sep. Purif. Technol., 2009, 68, 208–215 CrossRef CAS PubMed
.
- R. A. Kumbasar and O. Tutkun, Desalination, 2008, 224, 201–208 CrossRef CAS PubMed
.
- T. Kakoi, M. Goto and F. Nakashio, J. Membr. Sci., 1996, 120, 77–88 CrossRef CAS
.
- Y. Li, J. C. van Loon and R. R. Barefoot, Fresenius' J. Anal. Chem., 1993, 345, 467–470 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2015 |