Unique norbornene based triazole molecule for selective Fe(ii) sensing†
Abstract
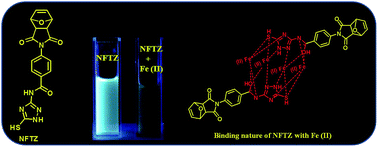
A triazole functionalized norbornene monomer (NFTZ) and its corresponding homopolymer (NFTZH) are synthesized. The unique fluorescence properties of the NFTZ molecule are due to the conjugation of two aromatic rings through an amide bond. The chelating behaviour of the NFTZ monomer is explored through the selective binding of Fe(II) in the presence of other metals. The MALDI-TOF analysis and Job’s plot clearly confirm the 1 : 2 mode of binding of NFTZ with Fe(II). 1H NMR and IR spectroscopy clearly confirm the unique amide-iminol tautomerisation.

 Please wait while we load your content...
Please wait while we load your content...