A tyrosine-rich peptide induced flower-like palladium nanostructure and its catalytic activity†
Abstract
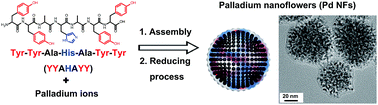
A specifically designed peptide, Tyr-Tyr-Ala-His-Ala-Tyr-Tyr (YYAHAYY), induced the formation of a flower-like palladium (Pd) nanostructure by controlling the size and shape of nanoparticles (NPs). The flower-shaped Pd NPs showed excellent catalytic activities in copper-free Sonogashira cross-coupling reaction in water.

 Please wait while we load your content...
Please wait while we load your content...