Ammonium ionic liquids with anions of natural origin†
Juliusz Pernak*a,
Bartosz Łęgosza,
Filip Walkiewicza,
Tomasz Klejdyszb,
Andrzej Borkowskic and
Łukasz Chrzanowskia
aDepartment of Chemical Technology, Poznan University of Technology, Poznan 60-965, Poland. E-mail: juliusz.pernak@put.poznan.pl; Tel: +48 61 665 3682
bInstitute of Plant Protection, National Research Institute, Poznan 60-318, Poland
cFaculty of Geology, University of Warsaw, Warsaw 02-089, Poland
First published on 24th July 2015
Abstract
This study was focused on presenting a new class of ionic liquids (ILs) with anions of natural origin. The salts were obtained by hydrolysis of defined triglycerides or edible vegetable oils (coconut oil and canola oil) by quaternary ammonium hydroxides. The basic physicochemical properties of the obtained products were studied and their microbial and deterrent activity was determined. Additionally, their susceptibility to biodegradation was evaluated. The described synthesis pathway utilizes vegetable oils and quaternary ammonium hydroxides as reactants in order to obtain ILs with anions of natural origin, which are characterized by specific biological activity. The majority of the prepared ILs exhibited excellent feeding deterrent properties and could be classified as readily biodegradable compounds. The employed method is characterized by low cost of substrates, high yields and high purity of the obtained products. It might potentially find application as a tool to synthesize environmentally friendly ILs with attractive properties for a wide range of applications.
Introduction
Ionic liquids (ILs) attract much scientific interest due to their properties and the possibility to obtain products with desired features.1–3 ILs are considered as less hazardous alternatives to volatile organic solvents, which is justified by their negligible vapour pressure. As a result, they are commonly described as ‘green solvents’, which adhere to the twelve principles of green chemistry.Furthermore, ILs have been intensely examined as compounds with unique and ‘designable’ properties, which can be adjusted by selecting appropriate ions in order to obtain a specific utility. These characteristics make ILs attractive for a wide range of applications in numerous fields, such as materials science, electrochemistry, catalysis, and medicinal chemistry.4 Recognition of the ILs' biological activity resulted in the establishment of the third generation of ILs (with targeted biological properties, combined with physical and chemical properties).5 They can be prepared directly from pharmaceutically-active ingredients6–10 and also from commonly used pesticides.11–16
Another potential application is associated with the control of crop pests, which are a worldwide cause of crop loss and an overall decrease of crop quality. Chemical compounds called antifeedants or feeding deterrents were introduced in order to prevent such pest activity. It is a class of substances, which temporarily or permanently limits the pests' ability to feed or reduces amount of food taken. There are several substances biosynthesized by plants, which do not take part in life processes. These substances, such as saponins, alkaloids, phenolics and terpenoids,17 are common components of essential oils and exhibit unique properties, including widespread deterrence. One of the most effective natural antifeedant found in Azadirachta indica is azadirachtin.18 This compound displays activity towards a broad spectrum of pest, therefore it is considered as one of the most efficient natural feeding deterrents. Unfortunately, the complicated chemical structure of azadirachtin, prevents its effective synthesis under laboratory conditions, therefore there is a strong need for effective and cheap alternatives. Such compounds can be obtained from substances commonly present in nature. This allows for obtaining products with lower toxicity compared to synthetic chemicals. Synthesis of terpenoid lactones from perillyl alcohols19 or dialkoxybenzenes from benzenediols20 as well as use of quinones,21 cinnamic aldehyde22 or even aminoacids23 may lead to non-toxic antifeedants. As a consequence, recent literature reports describe ILs as a new class of antifeedants.24–28
Among natural substances, oils (triglycerides) are most the important source of raw materials for industry. Global production of vegetable oils in 2013/14 reached an average of 162![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 million tons per annum and is estimated to reach 208
500 million tons per annum and is estimated to reach 208![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 690 million tons in 2023.29 A constant growth in this sector is expected. Their main sources are plants, such as rapeseed, soya, sunflower and olives. Despite the nutritional value of oils, only a relatively small part of their production yield is intended for the food industry. Oils undergo numerous processes, such as saponification, methanolysis, hydrogenation and hydrolysis, which result in a wide variety of products, such as surfactants, biofuels, plasticizers, lubricants, paints and coatings.30,31 Sodium or potassium carboxylates (soaps) are the oldest and most common surfactants, which are known for over 2000 years. The replacement of alkali ions in common soaps with tetraalkylammonium halides leads to the formation of tetraalkylammonium carboxylates.32–35 Recently, a highly water soluble surfactant based on choline carboxylate was described.36
690 million tons in 2023.29 A constant growth in this sector is expected. Their main sources are plants, such as rapeseed, soya, sunflower and olives. Despite the nutritional value of oils, only a relatively small part of their production yield is intended for the food industry. Oils undergo numerous processes, such as saponification, methanolysis, hydrogenation and hydrolysis, which result in a wide variety of products, such as surfactants, biofuels, plasticizers, lubricants, paints and coatings.30,31 Sodium or potassium carboxylates (soaps) are the oldest and most common surfactants, which are known for over 2000 years. The replacement of alkali ions in common soaps with tetraalkylammonium halides leads to the formation of tetraalkylammonium carboxylates.32–35 Recently, a highly water soluble surfactant based on choline carboxylate was described.36
The aim of the presented study was to investigate the properties, biological activity and biodegradability of alkyltrimethylammonium or dialkyldimethyl ammonium ILs with oils acting as anions of natural origin. The design focused on tetraalkylammonium carboxylates with symmetric cations, comprising either two or three methyl groups. This strategy was successful during previous studies,7,8,37,38 and the synthesized salts could be classified as ILs.
Results and discussion
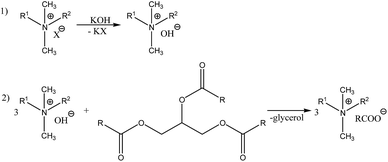
The studied quaternary ammonium salts were synthesized as shown in Scheme 1. Two defined triglycerides – glyceryl tristearate (TST) and trioleate (TOL) and two natural vegetable oils – canola (CAN) and coconut oil (COCO) were used as sources of anions. The selection of oils was based on the significant differences in their fatty acid profiles. Average molecular masses were calculated based on literature reports describing chromatographic analyses of oil compositions.39,40Reactions were conducted in 2-propanol as solvent and mixtures were heated under reflux. No transesterification reaction occurred under these conditions. Progress of the reaction was monitored by the decrease of the pH value. The termination of the reaction was determined by the stabilization of the pH value and then the solvent was evaporated under reduced pressure. This endothermic reaction led to the formation of quaternary ammonium salts and glycerol. The products were obtained with high yields (exceeding 95%) and are presented in Table 1. They were dried under vacuum (10 mbar) at 60 °C and stored over P4O10. The water content (measured by Karl-Fischer method) was determined to be less than 750 ppm. All of the obtained salts were liquids of high viscosity or waxes at ambient temperature and did not tend to crystallize, therefore they may be classified as ILs. Generally, ILs with saturated anion (TST, COCO) were waxes, whereas unsaturated anions caused products to act as liquids with high viscosity at ambient temperature. Additionally, the influence of the cation structure was observed. Some of the salts with two long alkyl chains (1–4) or large substituents, such as the phenyl group (5–8), were liquids at room temperature. Salts with only one long alkyl chain (9–12) were waxes. This may be caused by mutual arrangements between cation and anion as well as influences of alkyl chains among separate particles.
| Salt | R1 | R2 | Anion [RCOO] | Yield [%] | State |
|---|---|---|---|---|---|
| 1 | C10H21 | C10H21 | TST | 97 | Wax |
| 2 | C10H21 | C10H21 | TOL | 96 | Liquid |
| 3 | C10H21 | C10H21 | CAN | 98 | Liquid |
| 4 | C10H21 | C10H21 | COCO | 95 | Wax |
| 5 | CH2Ph | 60% C12H25, 40% C14H29 | TST | 95 | Wax |
| 6 | CH2Ph | 60% C12H25, 40% C14H29 | TOL | 95 | Liquid |
| 7 | CH2Ph | 60% C12H25, 40% C14H29 | CAN | 96 | Liquid |
| 8 | CH2Ph | 60% C12H25, 40% C14H29 | COCO | 98 | Wax |
| 9 | CH3 | C16H33 | TST | 95 | Wax |
| 10 | CH3 | C16H33 | TOL | 98 | Wax |
| 11 | CH3 | C16H33 | CAN | 98 | Wax |
| 12 | CH3 | C16H33 | COCO | 96 | Wax |
NMR spectra confirmed that the desired structures were formed. Protons in alkyl chains present in both cations and anions generated peaks in ranges from 0.82 to 1.01 ppm for methyl groups and between 1.23 and 1.38 ppm for methylene groups. Signals from protons of groups bonded directly to quaternary nitrogen atoms generated strong signals in range 3.18 to 3.34 ppm. Signals from protons of alkyl chains in α position to quaternary nitrogen were detected as peaks occurring from 3.25 to 3.46 ppm. Methylene groups in position β to nitrogen were observed as peaks in range from 1.59 to 1.78 ppm. Additionally, signals from anions were detected. Protons in β position to carbonyl group generated signals between 1.49 and 1.71 ppm, hydrogen atoms in position α to carbonyl were visible as signals from 2.11 to 2.29 ppm. Chemical shifts for protons in position α to double bond were observed between 2.00 and 2.12 ppm. Signals characteristic for double bonds in unsaturated anions were observed at values from 5.32 to 5.46 ppm.
The CHN analysis was not conducted for canolates 3, 7, 11 and cocoates 4, 8, 12 due to the fact that these compounds contained mixtures (the anion was a mixture of different fatty acids). The analysis was also not conducted for salts 5 and 6 as in this case the cation was a mixture. In the case of salts 3–8, 11 and 12 their purity was determined based on the TLC analysis in order to exclude the presence of glycerol.
The solubility test was conducted using popular solvents according to the method described by Vogel and Furniss.45 The results are presented in Table 2. ILs were insoluble in water, acetone and acetonitrile, soluble in methanol, chloroform and moderately in toluene. Solubility of ILs strongly depends on their chemical structure. Lack of solubility in water can be explained by the presence of long alkyl chains in both the cation and anion. Two alkyl chains in structures of didecyldimethylammonium ILs 1–4 led to insolubility in acetone and DMSO and moderate solubility in toluene, whereas ILs with only one alkyl substituent in the cation and a lower level of charge shielding were characterized by increased solubility (9–12 with hexadecyltrimethylammonium cation). Behaviour of benzalkonium ILs 5–8 with phenyl and alkyl substituents confirmed this statement. It was observed that the structure of the anion was also important. Saturated anions with long (TST) and short (COCO) alkyl chains caused a decrease of solubility. On the other hand, the presence of double bond in unsaturated anions (TOL and CAN) and their disposition resulted in an increase of solubility. All of the obtained ILs exhibited limited solubility in water (up to 500 ppm) except for ILs 9 and 12 what was proven during the anti-microbial activity studies. ILs containing a natural unsaturated anion (CAN) were unstable during storage. Their yellowish colour darkened and became orange. ILs with unsaturated anion obtained from defined triglyceride (TOL) were stable and they did not change their colour.
| IL | Solvent | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | |
| a A – water, B – methanol, C – DMSO, D – acetonitrile, E – acetone, F – ethyl acetate, G – chloroform, H – toluene, I – hexane; ‘+’ – complete solubility, ‘±’ – limited solubility, ‘−’ – insoluble. | |||||||||
| 1 | − | ± | − | − | − | − | ± | ± | − |
| 2 | − | + | − | − | − | − | + | ± | − |
| 3 | − | + | − | − | − | ± | + | + | ± |
| 4 | − | + | − | − | − | − | ± | ± | ± |
| 5 | − | + | − | − | − | − | + | ± | − |
| 6 | − | + | − | − | − | − | + | + | − |
| 7 | − | + | ± | − | − | ± | + | + | ± |
| 8 | − | + | ± | − | − | ± | + | ± | ± |
| 9 | − | + | ± | − | − | − | + | ± | − |
| 10 | − | + | + | − | ± | − | + | + | − |
| 11 | − | + | + | − | ± | − | + | + | − |
| 12 | − | + | ± | − | − | − | + | ± | − |
Phase transition temperatures and thermal stability of ILs were presented in Table 3. Each of the prepared salts melted below 100 °C. In case of didecyldimethylammonium ILs 1–4 no glass transition were observed. Crystallization temperatures for these ILs ranged from around −14 (2) up to 74 °C (1). Two liquid fractions were observed for IL 1: low with melting point at −1 °C and high with a melting point at 79 °C. Only one liquid fraction was observed for ILs 2 and 3 with a melting point of −7 and −1 °C, respectively. Three melting temperatures were observed for IL 4: −21, 11 and 36 °C. Among benzalkonium ILs only one salt was characterized by glass transition (7), which was observed at −47 °C. Three fractions were determined for IL 5: first with crystallization at 0, second at 33, and the last at 78 °C. The melting temperatures were at 1, 35 and 81 °C respectively. Three fractions were observed for IL 7 with crystallization temperatures at −38, 1 and 34 °C, and melting points at −34 next 3 and third at 28 °C. For hexadecyltrimethylammonium ILs 9–12 glass transition was observed between −27 °C for IL 10 and −16 °C for IL 11. Crystallization of IL 9 was first exhibited at around 11 °C and then at 40 °C, with melting points at 35 °C and 78 °C. In case of ILs 10 and 12 only one crystallization temperature and melting point were observed: 24 and 38 °C for IL 10 and 56 and 77 °C for IL 12, respectively. In case of IL 11 a broad crystallization region was observed from 6 °C (on cooling run) and two melting peaks were observed at 22 and 71 °C.
| IL | Tga [°C] | Tcb [°C] | Tmc [°C] | Tonset5d [°C] | Tonset50e [°C] | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||||
| a Glass transition.b Crystallization temperature.c Melting point.d Temperature Tonset to 5% mass loss.e Temperature from Tonset to 50% mass loss. | |||||||||
| 1 | — | −4 | 74 | — | −1 | 79 | — | 198 | 290 |
| 2 | — | −14 | — | — | −7 | — | — | 210 | 288 |
| 3 | — | −5 | — | — | −1 | — | — | 200 | 258 |
| 4 | — | −11 | 29 | — | −21 | 11 | 36 | 195 | 270 |
| 5 | — | 0 | 33 | 78 | 1 | 35 | 81 | 180 | 265 |
| 6 | — | −19 | 49 | — | 16 | 52 | — | 190 | 282 |
| 7 | −47 | −38 | 1 | 34 | −34 | 3 | 28 | 183 | 260 |
| 8 | — | 11 | 51 | — | 26 | 56 | — | 180 | 250 |
| 9 | — | 11 | 40 | — | 35 | 78 | — | 210 | 280 |
| 10 | −27 | 24 | — | — | 38 | — | — | 205 | 275 |
| 11 | −16 | 6 | — | — | 22 | 71 | — | 212 | 270 |
| 12 | — | 56 | — | — | 77 | — | — | 198 | 268 |
Generally, all of the prepared ILs were stable in the temperature range 180–210 °C (Table 3). Two-step thermal degradation processes were observed for all didecyldimethylammonium ILs. In case of IL 1 the first step ended at 369 °C and the second ranged from 369 to 440 °C with a mass loss 82% and 8%, respectively. First degradation of IL 2 occurred at 367 °C followed by a second step in the range of 367–484 °C. For IL 3 decompositions were observed to 314 °C for the first steep and the second between 314–497 °C. Decomposition of IL 4 occurred in two steps with the first one at 357 °C and the second ranging from 357 to 498 °C. ILs with benzalkonium cation exhibited multistep thermal decompositions. For IL 5 the first one ended at 260 °C, the second exhibited between 260–340 °C and the third ranged from 360 to 410 °C with mass losses of 38, 42 and 4% respectively. IL 6 showed two steps of decomposition – first exhibited between 190 and 367 °C with 82% mass loss and second ranging from 377 up to 440 °C. For ILs 7 and 8 only one step was observed in the range of 180–320 and 180–340 °C, respectively. In case of hexadecyltrimethylammonium ILs 9–12 multistep thermal decompositions were also observed. First decomposition step of IL 9 ended at 310 °C, the next step ranged from 320 to 420 °C with mass losses of 75 and 12%, for the first and second step respectively. Two steps were observed for IL 10: first at 310 °C with 78% mass loss and second in the range of 310 to 430 °C with 12% mass loss. Three steps were characterized for IL 11: the first step ranged from 210 to 310 °C, the second from 320 to 370 °C and the third from 370 to 440 °C, with mass losses of 73, 4 and 5% respectively. The first step of thermal decomposition for IL 12 ended at 309 °C (78%) and the next occurred between 320 and 400 °C (10%).
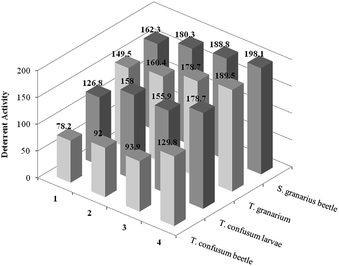
In the next step the feeding deterrent activity of the prepared ILs was examined. The obtained results were comparable to azadirachtin. The synthesized ILs were tested against beetles of Sitophilus granarius, Tribolium confusum (both beetles and larvae) and larvae of Trogoderma granarium. Feeding deterrent activity was estimated based on the amount of food consumed. Values of coefficients A (absolute coefficient of deterrence) and R (relative coefficient of deterrence) were calculated as follows:
where CC was the average weight of the food consumed in the control, TT was the average weight of the food consumed in the no-choice test and C and T expressed the average weights of the food consumed in the choice test. The sum of these two coefficients was used to evaluate the deterrent activity based on the following criteria: 200–151 very good, 150–101 good, 100–50 medium, <50 weak. Values of coefficients A and R were presented in Tables 4 and 5.
| IL | Sitophilus granarius (beetle) | Tribolium confusum (beetle) | ||||
|---|---|---|---|---|---|---|
| A | R | Det. act.b | A | R | Det. act.b | |
| a Azadirachtin.b Feeding deterrent activity. | ||||||
| 1 | 62.3 | 100.0 | Very good | 9.1 | 69.1 | Medium |
| 2 | 80.3 | 100.0 | Very good | 20.2 | 71.8 | Medium |
| 3 | 88.8 | 100.0 | Very good | 9.5 | 84.3 | Medium |
| 4 | 100.0 | 98.1 | Very good | 38.5 | 91.3 | Good |
| 5 | 35.1 | 100.0 | Good | 6.0 | 72.2 | Medium |
| 6 | 9.1 | 100.0 | Good | 47.9 | 100.0 | Good |
| 7 | 67.9 | 100.0 | Very good | 32.4 | 100.0 | Good |
| 8 | 45.3 | 100.0 | Good | 25.9 | 92.3 | Good |
| 9 | 8.5 | 94.6 | Good | 40.1 | 61.3 | Good |
| 10 | 5.7 | 96.8 | Good | 11.6 | 70.6 | Medium |
| 11 | 16.7 | 96.7 | Good | 40.9 | 78.0 | Good |
| 12 | 13.7 | 96.6 | Good | 41.8 | 55.9 | Medium |
| Stand.a | 74.3 | 100.0 | Very good | 85.0 | 100.0 | Very good |
| IL | Tribolium confusum (larvae) | Trogoderma granarium (beetle) | ||||
|---|---|---|---|---|---|---|
| A | R | Det. act.b | A | R | Det. act.b | |
| a Azadirachtin.b Feeding deterrent activity. | ||||||
| 1 | 31.5 | 95.3 | Good | 53.3 | 96.2 | Good |
| 2 | 64.6 | 93.4 | Very good | 66.4 | 94.0 | Very good |
| 3 | 57.1 | 98.8 | Very good | 80.3 | 98.3 | Very good |
| 4 | 78.7 | 100.0 | Very good | 92.5 | 97.0 | Very good |
| 5 | 43.0 | 100.0 | Good | 48.3 | 100.0 | Good |
| 6 | 40.2 | 92.6 | Good | 63.5 | 87.6 | Very good |
| 7 | 64.9 | 100.0 | Very good | 82.4 | 98.4 | Very good |
| 8 | 82.4 | 100.0 | Very good | 85.1 | 98.0 | Very good |
| 9 | 54.6 | 69.3 | Good | 61.2 | 34.5 | Medium |
| 10 | 66.6 | 84.1 | Good | 78.1 | 87.6 | Very good |
| 11 | 92.7 | 95.0 | Very good | 53.6 | 58.9 | Good |
| 12 | 72.8 | 97.9 | Very good | 50.9 | 64.6 | Good |
| Stand.a | 88.4 | 100.0 | Very good | 94.2 | 100.0 | Very good |
Most of the synthesized ILs can be described as good or very good feeding deterrents. Moreover, the results were strongly influenced by the tested organisms. Didecyldimethylammonium ILs 1–4 were as efficient as azadirachtin (±10%) or even better towards Sitophilus granarius beetles. In case of Tribolium confusum, both beetles and larvae, benzalkonium ILs 5–8 exhibited the highest efficiency. Feeding deterrent activity towards Trogoderma granarium was comparable for these two groups of ILs. Hexadecyltrimethylammonium ILs 9–10 were most efficient towards Tribolium confusum larvae, in other cases their results significantly differed from other tested compounds, yet they still remained good deterrents. Tribolium confusum beetle was the most resistant species to the tested ILs – differences between tested ILs and azadirachtin were highest.
Influence of ions on feeding deterrent activity of obtained ILs is presented in Schemes 2 and 3. The role of either cation and anion was clearly visible. Proper selection of cation and anion may potentially increase the efficiency of ILs compared to the reference standard – azadirachtin.
 | ||
| Scheme 3 Role of cation in feeding deterrence activity for ILs with anion of natural origin (COCO), cation: didecyldimethylammonium 4, benzalkonium 8 and hexadecyltrimethylammonium 12. | ||
Comparison of the structures of synthesized ILs (Scheme 1) with the azadirachtin structure (Scheme 4) led to the conclusion that the employed strategy of searching for new feeding deterrents is justified.
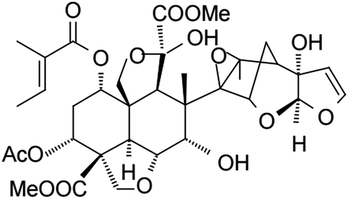 | ||
| Scheme 4 Chemical structure of azadirachtin A.18 | ||
High activity of quaternary ammonium halides against microorganisms was the basis for testing the synthesized ILs in this field. The influence of introducing an organic anion to the structure was determined. Anti-microbial activity was investigated against cocci, rods and fungi by specifying MIC/MBC and MIC/MFC values, which were presented in Tables 6 and 7. ILs 9 and 12 were eliminated from further studies due to limited solubility in water (approx. 500 ppm).
| Strain | 1 | 2 | 3 | 4 | [DDA][Cl] | |
|---|---|---|---|---|---|---|
| a In ppm. | ||||||
| M. luteus | MIC | <0.1 | 0.1 | 1.0 | 0.1 | 0.1 |
| MBC | <0.1 | 0.1 | 2.0 | 2.0 | 0.1 | |
| S. aureus | MIC | <0.1 | 1.0 | 2.0 | 0.5 | 0.1 |
| MBC | 0.2 | 1.0 | 2.0 | 1.0 | 0.2 | |
| S. epidermidis | MIC | <0.1 | 0.1 | 1.0 | 0.2 | 0.1 |
| MBC | 1.0 | 1.0 | 4.0 | 4.0 | 0.2 | |
| E. faecium | MIC | 2.0 | 1.0 | 8.0 | 1.0 | 0.1 |
| MBC | 4.0 | 4.0 | 8.0 | 4.0 | 0.2 | |
| M. catarrhalis | MIC | 0.1 | 1.0 | 2.0 | 0.2 | 0.1 |
| MBC | 0.2 | 1.0 | 4.0 | 1.0 | 0.2 | |
| E. coli | MIC | 8.0 | 0.5 | 1.0 | 8.0 | 0.1 |
| MBC | 62.0 | 0.5 | 2.0 | 62.0 | 0.2 | |
| B. subtilis | MIC | 0.1 | 0.5 | 2.0 | 0.5 | 1.0 |
| MBC | 0.2 | 2.0 | 4.0 | 1.0 | 2.0 | |
| S. marcescens | MIC | >500 | 31.0 | 1.0 | >500 | 0.1 |
| MBC | >500 | >500 | 4.0 | >500 | 0.5 | |
| P. vulgaris | MIC | 62.0 | 125.0 | 31.0 | 31.0 | 4.0 |
| MBC | 62.0 | 500.0 | 125.0 | 62.0 | 8.0 | |
| P. aeruginosa | MIC | 125.0 | 250.0 | 125.0 | 250.0 | 16.0 |
| MBC | >500 | 500.0 | 125.0 | 500.0 | 31.0 | |
| C. albicans | MIC | 31.0 | 31.0 | 4.0 | 8.0 | 4.0 |
| MFC | 62.0 | 31.0 | 4.0 | 16.0 | 4.0 | |
| R. rubra | MIC | 62.0 | 8.0 | 4.0 | 31.0 | 4.0 |
| MFC | 62.0 | 31.0 | 8.0 | 31.0 | 4.0 | |
| Strain | 5 | 6 | 7 | 8 | [BA][Cl] | 10 | 11 | [CET][Br] | |
|---|---|---|---|---|---|---|---|---|---|
| a In ppm. | |||||||||
| M. luteus | MIC | 2.0 | 0.5 | 0.1 | 2.0 | 0.1 | 0.1 | 2.0 | 0.5 |
| MBC | 2.0 | 1.0 | 0.1 | 4.0 | 0.1 | 4.0 | 16.0 | 1.0 | |
| S. aureus | MIC | 2.0 | 0.5 | 1.0 | 2.0 | 0.2 | 2.0 | 2.0 | 1.0 |
| MBC | 4.0 | 4.0 | 4.0 | 4.0 | 2.0 | 2.0 | 8.0 | 1.0 | |
| S. epidermidis | MIC | 2.0 | 0.5 | 4.0 | 2.0 | 2.0 | 2.0 | 8.0 | 0.5 |
| MBC | 4.0 | 2.0 | 4.0 | 4.0 | 2.0 | 4.0 | 31.0 | 2.0 | |
| E. faecium | MIC | 4.0 | 4.0 | 4.0 | 2.0 | 1.0 | 4.0 | 8.0 | 1.0 |
| MBC | 16.0 | 16.0 | 16.0 | 8.0 | 1.0 | 31.0 | 8.0 | 2.0 | |
| M. catarrhalis | MIC | 2.0 | 2.0 | 1.0 | 2.0 | 1.0 | 0.5 | 4.0 | 1.0 |
| MBC | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 1.0 | 4.0 | 1.0 | |
| E. coli | MIC | 2.0 | 0.5 | 2.0 | 2.0 | 1.0 | 1.0 | 4.0 | 0.5 |
| MBC | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 1.0 | 16.0 | 4.0 | |
| B. subtilis | MIC | 2.0 | 1.0 | 2.0 | 2.0 | 1.0 | 2.0 | 31.0 | 1.0 |
| MBC | 2.0 | 2.0 | 2.0 | 2.0 | 1.0 | 4.0 | 31.0 | 2.0 | |
| S. marcescens | MIC | 62.0 | >500 | 4.0 | 62.0 | 2.0 | >500 | >500 | 31.0 |
| MBC | 500.0 | >500 | 8.0 | 500.0 | 4.0 | >500 | >500 | 62.0 | |
| P. vulgaris | MIC | 500.0 | >500 | 125.0 | 500.0 | 8.0 | >500 | >500 | 31.0 |
| MBC | 500.0 | >500 | 250.0 | >500 | 16.0 | >500 | >500 | 62.0 | |
| P. aeruginosa | MIC | 250.0 | >500 | 500.0 | 250.0 | 31.0 | >500 | >500 | 125.0 |
| MBC | 500.0 | >500 | >500 | 250.0 | 125.0 | >500 | >500 | >500 | |
| C. albicans | MIC | 16.0 | 16.0 | 8.0 | 8.0 | 2.0 | 4.0 | 4.0 | 1.0 |
| MFC | 500.0 | 62.0 | 16.0 | 31.0 | 4.0 | 16.0 | 31.0 | 1.0 | |
| R. rubra | MIC | 31.0 | 8.0 | 4.0 | 31.0 | 2.0 | 4.0 | 2.0 | 0.5 |
| MFC | 125.0 | 31.0 | 4.0 | 125.0 | 2.0 | 31.0 | 16.0 | 0.5 | |
Quaternary ammonium salts – didecyldimethylammonium chloride [DDA][Cl], benzalkonium chloride [BA][Cl] and hexadecyltrimethylammonium bromide [CET][Br] – were used as reference compounds. Values presented in Tables 6 and 7 indicate that the effect of introducing an organic anion into the IL structure strongly depended on the tested species. The synthesized ILs were active against cocci as compared to corresponding halides. Introduction of an organic cation into the structures of ILs with all three kinds of cation did not result lower their activity. Among didecyldimethylammonium ILs the MIC/MBC values calculated for IL 1 indicated that activity towards M. luteus, S. aureus and S. epidermidis was higher than the corresponding halide. ILs 2 and 4 were the most efficient against E. faecium. In case of rods tested for ILs 1–8, 10 and 11, S. marcescens, P. vulgaris and P. aeruginosa presented highest tolerance to tested ILs with high MIC/MBC values.
It should be assumed that ILs 1–8, 10 and 11 were not active against those microbial species. The exceptions were two ILs with CAN anion (3 and 7), which proved to be highly effective against S. marcescens.
However, as can be seen from Table 6 for M. catarrhalis, E. coli and B. Subtilis, the highest efficiency was observed for ILs 1 and 2, which remained at a similar level to reference compound. Additionally, the tested fungi exhibited higher tolerance to the synthesized ILs than the original halide. IL 3 was most efficient against C. albicans and R. rubra. Similar observations were made for benzalkonium ILs (Table 7). Introduction of the natural anion allowed to retain the activity against all cocci species. IL 6 was the most efficient, presenting lowest MIC/MBC values for S. aureus and S. epidermidis. IL 7 with the CAN anion was characterized as most active towards cocci as well as fungi. Only two hexadecyltrimethylammonium ILs 10 and 11 were tested. Activity of ILs with unsaturated anions remained similar to the reference halides for cocci. Slight decrease in efficiency of 10 and 11 was observed towards fungi.
Every tested species presented different tolerance to the examined ILs. In general, it was observed that ILs 1–4 with didecyldimethylammonium cation were most efficient. Highest activity among obtained ILs was observed for IL 1. The tested ILs were not effective against S. marcescens, P. vulgaris and P. aeruginosa. The loss of activity compared to the reference compounds might be explained by the large ILs anion.
The results obtained during biodegradation tests revealed that the studied ILs exhibited different susceptibility to mineralization processes (Table 8). The majority of the ILs (8 out of 12) were readily biodegradable (biodegradation efficiency > 80%), one was dissipated to a lesser extent (biodegradation efficiency < 40%), whereas the remaining ILs (3 out of 12) were resistant to biodegradation (biodegradation efficiency < 10%). The differences in biodegradability correspond well with the structural differences among the studied ILs and allow for distinction of two major effects. The first effect is associated with the influence of the cation. All four hexadecyltrimethylammonium ILs reached the highest biodegradation efficiency (100% for 9, 10, 11 and 90% for 12) and could be classified as readily biodegradable, whereas the biodegradability of didecyldimethylammonium and benzalkonium ILs was diversified. The second effect may be attributed to the effect of the anion. Among ILs 1–8 the highest biodegradation efficiency was observed when oleate or canola oil were used as anions (87% and 84% for 2 and 3 with the didecyldimethylammonium cation, 100% for both 6 and 7 with the benzalkonium cation, accordingly). Considerably lower biodegradability was observed in case of ILs comprising coconut oil as anions (36% for 4 with the didecyldimethylammonium cation, 12% for 8 with the benzalkonium cation, accordingly), whereas the mineralization of ILs with stearate anions was lowest (5% for 1 with the didecyldimethylammonium cation, 8% for 5 with the benzalkonium cation, accordingly). On the basis of the obtained results, it can be established that the use of alkyltrimethylammonium cation results in increased biodegradability compared to dialkyldimethylammonium-based cation. Additionally, the analysis of biodegradability of dialkyldimethylammonium ILs leads to the conclusion that the use of unsaturated oil components as anions (oleate or canola oil) results in improved biodegradability as opposed to ILs with saturated anions (stearate or coconut oil).
| IL | Biodegradation after 28 days [%] | Time window between 10 and 60% [days] | Classification |
|---|---|---|---|
| 1 | 5 ± 1 | — | Not readily biodegradable |
| 2 | 87 ± 7 | 8 | Readily biodegradable |
| 3 | 84 ± 8 | 9 | Readily biodegradable |
| 4 | 36 ± 4 | — | Not readily biodegradable |
| 5 | 8 ± 2 | — | Not readily biodegradable |
| 6 | 100 ± 9 | 2 | Readily biodegradable |
| 7 | 100 ± 12 | 2 | Readily biodegradable |
| 8 | 12 ± 3 | — | Not readily biodegradable |
| 9 | 100 ± 8 | 2 | Readily biodegradable |
| 10 | 100 ± 10 | 2 | Readily biodegradable |
| 11 | 100 ± 11 | 3 | Readily biodegradable |
| 12 | 90 ± 10 | 7 | Readily biodegradable |
Based on studies focused on the biodegradability of ILs, it can be established that their susceptibility to biodegradation processes depends on three fundamental structural factors: the core of the cation, the type of cation substituents and the type of the anion.41 The core of the cation was the same for all ILs tested in the framework of this study (tetraalkylammonium), therefore it can be concluded that the latter two factors influenced the biodegradability. The fundamental effect of the type and number of cation substituents on the biodegradation of ILs was confirmed in numerous studies.42,43 Overall, the increased number of long-chain alkyl substituents results in a decrease of the biodegradability. This observation correspond well with the results of this study, since higher biodegradability was observed for ILs comprising the alkyltrimethylammonium cation (9–12) compared to dialkyldimethylammonium-based ILs (1–8). The biodegradation rate of ILs comprising tetrabutylammonium cation and different natural organic acids was studied by Ferlin et al. 2013.44 The authors reported that the overall biodegradability of the obtained ILs was below 25% therefore none could be classified as readily biodegradable. This contrasts with the results presented in this study, however the differences may be a result of structural differences between the cations as well as anions. On the other hand, the results obtained by Ferlin et al. suggest that differences in the biodegradability of ILs may also be caused by the use of different anions. This is in accordance with the results presented in this study, as notable differences between ILs comprising the same cation and different anions could be observed for dialkyldimethylammonium-based salts (high biodegradability in case of oleate or canola oil and low biodegradability in case of stearate or coconut oil). This confirms that the influence of the anion is also an important factor when considering the biodegradability of ILs.
Experimental
Materials
Glyceryl tristearate (97%) and oleate (98%), vegetable oils (rapeseed, coconut) and quaternary ammonium halides: didecyldimethylammonium chloride [DDA][Cl] (98%), benzalkonium chloride [BA][Cl] (60% C12, 40% C14; 95%), hexadecyltrimethylammonium bromide [CET][Br] (98%), potassium hydroxide and all solvents were supplied by Sigma-Aldrich and POCh, and used as obtained.Synthesis
Quaternary ammonium halides (0.03 mol) were placed in round-bottomed flasks with 50 mL of 2-propanol. Next, a stoichiometric amount of potassium hydroxide dissolved in 2-propanol was added. The mixture was stirred at ambient temperature for 10 minutes. In the next step, the precipitated inorganic salt was filtered off and 0.01 mol of triglyceride was added. Reaction was monitored by pH electrode placed in flask. Reagents were heated under reflux until the pH reached constant value. The solvent was evaporated under vacuum at 60 °C and then the flask content was dissolved in 50 mL of chloroform and placed in a separator. The organic layer was then washed three times with 20 mL of distilled water. After separation of phases the organic layer was poured to a flask and the solvent was evaporated under reduced pressure. The product was then dried under vacuum (10 mbar) at 60 °C for 24 h.Analysis
1H NMR spectra were recorded on a Mercury Gemini 300 spectrometer operating at 300 MHz with TMS as the internal standard. 13C NMR spectra were obtained with the same instrument at 75 MHz. Water content was determined using an Aquastar volumetric Karl Fischer titration with composite 5 solution as the titrant and anhydrous methanol as a solvent. Purity of the synthesized salts was determined by TLC method and analysis of NMR spectra. Elemental analyses were performed at the Adam Mickiewicz University, Poznan (Poland).Solubility
The solubility of prepared ILs was determined according to Vogel's Textbook of Practical Organic Chemistry.45 Popular representative solvents were chosen and ranked in descending order of Snyder polarity index value (water – 9.0, methanol – 6.6, DMSO – 6.5, acetonitrile – 6.2, acetone – 5.1, ethyl acetate – 4.3, chloroform – 4.1, toluene – 2.3, hexane – 0.0). The term ‘complete solubility’ refers to ILs, which were dissolved (0.1 g of IL) in 1 mL of solvent, while the term ‘limited solubility’ means that the IL was dissolved in 3 mL of solvent. The term ‘insoluble’ was used to describe no solubility of 0.1 g of IL in 3 mL of solvent. Tests were conducted at 20 °C under ambient pressure.Thermal stability
Thermal transition temperatures were determined by DSC, with a Mettler Toledo Stare DSC1 (Leicester, UK) unit, under nitrogen. Samples (between 5 and 15 mg) were placed in aluminum pans and heated from 25 to 120 °C at a heating rate of 10 °C min−1, cooled with an intracooler at a cooling rate of 10 °C min−1 to −100 °C, then heated again to 120 °C. Thermogravimetric analysis was performed using a Mettler Toledo Stare TGA/DSC1 unit (Leicester, UK), under nitrogen. ILs (between 2 and 10 mg) were placed in aluminum pans and heated from 30 to 450 °C at a heating rate of 10 °C min−1. Two cycles at different heating/cooling rates (10 and 2 °C min−1) were conducted.Feeding deterrent activity
The bioassay experiments were conducted with Tribolium confusum Duv. (larvae and adults), Sitophilus granaries L. (adults), and Trogoderma granarium Ev. (larvae). The insects were grown on a wheat grain or whole-wheat meal diet in laboratory colonies, which were maintained at 26 ± 1 °C and 60 ± 5% relative humidity. Choice and no-choice tests for insect-feeding were conducted following a previously described procedure.46 Wheat wafers discs (1 cm in diameter, 1 mm thick) were saturated by dipping either in ethanol only (control) or in a solution of the studied ILs (1%) in ethanol to be tested. After evaporation of the solvent (30 min of air-drying) the wafers were weighted and offered to the insects in plastic boxes as the sole food source for 5 days. The feeding of insects was recorded under three sets of conditions: (1) on two control discs (CC), (2) on a choice between one treated disc (T) and one control disc (C; choice test), and (3) on two treated discs (TT; no-choice test). Each of the three experiments was repeated five times with 3 adults of Sitophilus granaries, 20 adults and 10 larvae of Trogoderma granarium. The number of individual insects depended on the intensity of their food consumption. The adults used for experiments were unsexed, 7–10 days old, and the larvae were 5–30 days old. After 5 days the discs were weighted and the average weight of eaten food was calculated.Anti-microbial activity
Anti-microbial activity was determined by the tube dilution method. A series of ammonium salts dilutions was prepared on Müller-Hinton broth medium (bacteria) or Sabouraud broth medium (fungi). A suspension of the microorganisms, prepared from 24 h cultures of bacteria in the Müller-Hinton broth medium and from 48 h cultures in the Sabouraud agar medium for fungi, at a concentration of 106 cfu mL−1, were added to each dilution in a 1 + 1 ratio. Growth (or lack of growth) of the microorganisms was determined visually after incubation for 24 h at 37 °C (bacteria) or 48 h at 28–30 °C (fungi). The lowest concentration at which there was no visible growth (turbidity) was taken as the MIC. Then, one loopful from each tube was cultured on an agar medium with inactivates (0.3% lecithin, 3% polysorbate 80 and 0.1% L-cysteine) and incubated for 48 h at 37 °C (bacteria) or for 5 days at 28–30 °C (fungi). The lowest concentration of the salt supporting no colony formation was defined as the MBC and MFC. The following microorganisms were used during the tests: standard strains representative of cocci: Micrococcus luteus ATCC 9341, Staphylococcus aureus ATCC 6538, Staphylococcus epidermidis ATCC 12228, Enterococcus faecium ATCC 49474; rods: Moraxella catarrhalis ATCC 25238, Escherichia coli NCTC 8196, Bacillus subtilis ATCC 6633, Serratia marcescens ATCC 8100, Proteus vulgaris NCTC 4635, Pseudomonas aeruginosa ATCC 15442 and fungi: Candida albicans ATCC 10231, Rhodotorula rubra (Demml 1889, Lodder 1934). Standard strains were supplied by the National Collection of Type Cultures (NCTC) London and American Type Culture Collection (ATCC). Rhodotorula rubra was obtained from the Department of Pharmaceutical Bacteriology, K. Marcinkowski University of Medical Sciences, Poznan.Ready biodegradability according to OECD 301 F test – manometric respirometry
The test was performed according to OECD guideline for 301 F test. The oxygen uptake and CO2 production was determined every 5 h for 28 days using the Micro-Oxymax Respirometer (Columbus Instruments, USA). The activated sludge was collected from a local municipal wastewater treatment plant (Czajka, Warsaw, Poland). Prior to use, the activated sludge was aerated for 7 days in mineral medium which was also used for subsequent tests. The mineral medium consisted of KH2PO4 – 85 mg L−1, K2HPO4 – 220 mg L−1, Na2HPO4·2H2O – 220 mg L−1, NH4CL – 17 mg L−1, CaCl2·2H2O – 37 mg L−1, MgSO4·2H2O – 23 mg L−1, FeCl3 – 0.25 mg L−1. The measured pH was at 7.2. The test was performed in brown glass bottles (100 mL Simax) containing mineral medium, inoculum (cell density at approx. 106 cells per mL determined with plastic Paddle Tester for aerobic bacteria, Hach, USA) and tested ILs at a concentration of approx. 10–30 mg L−1, which was equal to 100 mg L−1 of Theoretical Oxygen Demand (ThOD, calculated based on equation CcHhNnOoPp).Allylthiourea (1.16 mg L−1) was added to inhibit nitrification. All samples were analyzed in triplicates together with controls (sodium benzoate without inoculum, tested substances without inoculum) and blanks (medium and inoculum without tested substances). Gas tight flasks were connected with the respirometric system and incubated in the dark at 22 °C for 28 days. The CO2 sensor range spanned the volume of 0–15%. Sensitivity of the respirometer was at 0.2 μL h−1. The biodegradation efficiency was calculated based on the oxygen uptake in each bottle (measured automatically by the Micro-Oxymax) and corrected for the oxygen demand of the blank, with the respect to the theoretical oxygen demand and the amount of ILs tested.
13C NMR (DMSO-d6) δ (ppm): 13.9; 22.5; 25.2; 26.2; 27.0; 29.1; 31.7; 39.0; 51.2; 63.4; 179.9.
Elemental analysis calc. (%) for C40H83NO2 (610.09 g mol−1): C 78.75, H 13.71, N 2.30. Found: C 79.12, H 14.00, N 2.40.
13C NMR (DMSO-d6) δ (ppm): 13.8; 21.5; 22.3; 25.0; 26.9; 29.0; 31.5; 38.7; 51.0; 63.4; 129.6; 179.9.
Elemental analysis calc. (%) for C40H81NO2 (608.08 g mol−1): C 79.01, H 13.43, N 2.30. Found: C 79.16, H 13.63, N 2.41.
13C NMR (CDCl3) δ (ppm): 14.0; 22.1; 25.8; 26.6; 26.8; 28.7; 31.3; 39.0; 51.2; 62.7; 129.7; 175.4.
13C NMR (CDCl3) δ (ppm): 13.9; 22.5; 25.2; 26.2; 28.9; 29.1; 31.7; 39.1; 51.1; 63.3; 179.8.
13C NMR (DMSO-d6) δ (ppm): 14.0; 21.7; 22.6; 26.2; 27.0; 29.2; 31.8; 38.9; 50.0; 63.7; 67.5; 126.8; 127.6; 129.0; 130.4; 132.9; 173.3.
13C NMR (DMSO-d6) δ (ppm):14.0; 21.7; 22.6; 26.2; 27.1; 29.5; 31.8; 34.6; 39.1; 49.7; 63.8; 67.6; 127.5; 129.1; 130.5; 133.0; 173.3.
13C NMR (CDCl3) δ (ppm): 14.0; 22.6; 25.3; 26.2; 27.2; 29.5; 31.8; 34.6; 39.2; 49.6; 63.1; 67.4; 127.6; 129.0; 129.8; 130.4; 133.0; 179.9.
13C NMR (CDCl3) δ (ppm): 14.0; 22.5; 25.2; 26.2; 27.1; 29.4; 29.4; 31.7; 34.6; 39.2; 49.5; 63.1; 67.5; 127.5; 129.0; 133.0; 179.9.
13C NMR (DMSO-d6) δ (ppm):14.0; 22.6; 24.9; 26.1; 29.6; 31.8; 38.9; 53.2; 66.8; 173.8.
Elemental analysis calc. (%) for C37H77NO2 (568.01 g mol−1): C 78.24, H 13.66, N 2.47. Found C 78.03, H 13.29, N 2.79.
13C NMR (DMSO-d6) δ (ppm): 14.0; 22.6; 23.1; 24.8; 26.9; 29.6; 31.8; 38.7; 53.1; 72.7; 129.7; 180.4.
Elemental analysis calc. (%) for C37H75NO2 (566.00 g mol−1): C 78.52, H 13.36, N 2.47. Found C 78.71, H 13.71, N 2.72.
13C NMR (CDCl3) δ (ppm): 13.9; 22.5; 25.2; 26.1; 27.0; 29.5; 31.8; 39.0; 53.0; 66.6; 129.7; 179.9.
13C NMR (CDCl3) δ (ppm): 13.9; 22.5; 25.2; 26.1; 27.0; 29.5; 31.7; 39.0; 52.9; 66.6; 179.8.
Conclusions
This study presents a new method of obtaining ILs from natural sources. It is focused on the use of natural vegetable oils as anionic moieties for synthesis of biologically active ILs. The salts were synthesized with high yield and purity. It was established that both the physicochemical properties (solubility, phase transition temperatures and thermal stability) and activity of the studied ILs depend on their chemical structures. The feeding deterrent activity was very good to good in the case of Sitophilus granarius (beetle), Tribolium confusum (larvae), Trogoderma granarium (beetle) and medium to good in case of Tribolium confusum (beetle). Comparison of anti-microbial properties of the obtained ILs and precursor compounds revealed that the studied ILs were characterized by similar activity. A species-specific diversity in the efficiency of the ILs could be observed during both the feeding deterrent activity and anti-microbial activity assays, which is most likely associated with their structural differences. Furthermore, the biodegradation tests confirmed that most of the ILs exhibited high biodegradation efficiency (>80%) and may be classified as readily biodegradable. The tests revealed a notable influence of the cations (ILs with alkyltrimethylammonium cation were more biodegradable compared to ILs with dialkyldimethylammonium cation), followed by the effect of anions (ILs with unsaturated oleate and canola oil anions were more biodegradable compared to ILs with saturates stearate and coconut oil anions). These findings confirm that the described method may be successfully used for synthesis of ILs from natural products and elucidates valuable guidelines for the design of efficient products with attractive properties for a wide range of applications.Acknowledgements
This work was supported by National Science Center, Poland, (grant No. DEC-2012/07/B/ST5/00806).Notes and references
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH VerlagGmbh& Co. KGaA, Weinheim, 2nd edn, 2008 Search PubMed.
- H. Olivier-Bourbigou, L. Magna and P. Morvan, Appl. Catal., A, 2010, 373, 1 CrossRef CAS PubMed.
- J. P. Hallet and T. Welton, Chem. Rev., 2011, 111, 3508 CrossRef PubMed.
- M. Smiglak, J. M. Pringle, X. Lu, L. Han, S. Zhang, H. Gao, D. R. MacFarlane and R. D. Rogers, Chem. Commun., 2014, 50, 9228 RSC.
- W. L. Hough, M. Smiglak, H. Rodriguez, R. P. Swatloski, S. K. Spear, D. T. Daly, J. Pernak, J. E. Grisel, R. D. Carliss, D. M. Soutullo, J. H. Davis and R. D. Rogers, New J. Chem., 2007, 31, 1429 RSC.
- W. L. Hough and R. D. Rogers, Bull. Chem. Soc. Jpn., 2007, 80, 2262 CrossRef CAS.
- J. Stoimenovski, D. R. MacFarlane, K. Bica and R. D. Rogers, Pharm. Res., 2010, 27, 521 CrossRef CAS PubMed.
- K. Bica, C. Rijksen and M. Nieuwenhuyzen, Phys. Chem. Chem. Phys., 2010, 12, 2011 RSC.
- P. C. A. G. Pinto, D. M. G. P. Ribeiro, A. M. O. Azevedo, D. J. Vanessa, E. Cunha, K. Bica, M. Vasiloiu, S. Reis and M. L. M. F. S. Saraiva, New J. Chem., 2013, 37, 4095 RSC.
- I. M. Marrucho, L. C. Branco and L. P. N. Rebelo, Annu. Rev. Chem. Biomol. Eng., 2014, 5, 527 CrossRef CAS PubMed.
- J. Pernak, A. Syguda, D. Janiszewska, K. Materna and T. Praczyk, Tetrahedron, 2011, 67, 4838 CrossRef CAS PubMed.
- K. Bica, L. R. Cooke, C. Rijksen and R. D. Rogers, Green Chem., 2011, 13, 2344 RSC.
- J. Pernak, K. Czerniak, M. Niemczak, Ł. Chrzanowski, Ł. Ławniczak, P. Fochtman, K. Marcinkowska and T. Praczyk, New J. Chem., 2015, 39, 5715 RSC.
- O. A. Cojocaru, J. L. Shamshina, G. Gurau, A. Syguda, T. Praczyk, J. Pernak and R. D. Rogers, Green Chem., 2013, 15, 2110 RSC.
- J. Pernak, M. Niemczak, R. Giszter, J. L. Shamshina, G. Gurau, O. Andreea Cojocaru, T. Praczyk, K. Marcinkowska and R. D. Rogers, ACS Sustainable Chem. Eng., 2014, 2, 2845 CrossRef CAS.
- J. Pernak, M. Niemczak, J. L. Shamshina, G. Gurau, G. Głowacki, T. Praczyk, K. Marcinkowska and R. D. Rogers, J. Agric. Food Chem., 2015, 13, 3357 CrossRef PubMed.
- J. Nawrot and J. Hermatha, Phytochem. Rev., 2012, 11, 543 CrossRef CAS.
- OECD/Food and Agriculture Organization of the United Nations, OECD-FAO Specifications and Evaluations for Azadirachtin, http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Specs/azadirachtin2006.pdf, 27.05.2015.
- E. Paruch, Z. Ciniuk, J. Nawrot and C. Wawrzeńczyk, J. Agric. Food Chem., 2000, 48, 4973 CrossRef CAS PubMed.
- Y. Akhtar, Y. Yu, M. B. Isman and E. Plettner, J. Agric. Food Chem., 2010, 58, 4983 CrossRef CAS PubMed.
- Y. Akhtar, M. B. Isman, L. A. Niehaus, C. Lee and H. Lee, Crop Prot., 2012, 31, 8 CrossRef CAS PubMed.
- B. K. Babbar, J. Kaur, N. Singla and A. Kaur Mahal, Crop Prot., 2015, 67, 235 CrossRef CAS PubMed.
- M. Hilker, C. Haberlein, U. Trauer, M. Bunnige, M. O. Vicentini and S. Schulz, ChemBioChem, 2010, 11, 1720 CrossRef CAS PubMed.
- J. Pernak, A. Syguda, I. Mirska, A. Pernak, J. Nawrot, A. Prądzyńska, S. T. Griffin and R. D. Rogers, Chem.–Eur. J., 2007, 13, 6817 CrossRef CAS PubMed.
- W. L. Hough-Troutman, M. Smiglak, S. Griffin, W. M. Reichert, I. Mirska, J. Jodynis-Liebert, T. Adamska, J. Nawrot, M. Stasiewicz, R. D. Rogers and J. Pernak, New J. Chem., 2009, 33, 26 RSC.
- J. Pernak, K. Wasiński, T. Praczyk, J. Nawrot, A. Cieniecka-Rosłonkowicz, F. Walkiewicz and K. Materna, Sci. China: Chem., 2012, 55, 1532 CrossRef CAS.
- J. Pernak, J. Nawrot, M. Kot, B. Markiewicz and M. Niemczak, RSC Adv., 2013, 3, 25019 RSC.
- B. Markiewicz, A. Sznajdrowska, Ł. Chrzanowski, Ł. Ławniczak, A. Zgoła-Grzeskowiak, K. Kubiak, J. Nawrot and J. Pernak, New J. Chem., 2014, 38, 3146 RSC.
- OECD/Food and Agriculture Organization of the United Nations, OECD-FAO Agricultural Outlook 2014, OECD Publishing, 2014, ISBN 978-92642-1174-2 Search PubMed.
- Ullmann's Encyclopedia of Industrial Chemistry, 6th ed., Fats and Fatty Oils, A. Thomas, 1–73, 2013.
- Bailey's Industrial Oil and Fat Products, ed. F. Shahidi, John Wiley & Sons, 6th edn, 2005, vol. 5, ISBN: 978-0-471-38460-1 Search PubMed.
- Z. J. Yu, X. Zhang and G. Xu, J. Phys. Chem., 1990, 94, 3675 CrossRef CAS.
- M. Jansson, A. Jönsson, P. Li and P. Stilbs, Colloids Surf., 1991, 59, 387 CrossRef CAS.
- R. Zana, Langmuir, 2004, 20, 5666 CrossRef CAS.
- D. Parmentier, S. J. Metz and M. C. Kroon, Green Chem., 2013, 15, 205 RSC.
- R. Klein, D. Touraud and W. Kunz, Green Chem., 2008, 10, 433 RSC.
- J. Pernak, M. Smiglak, S. T. Griffin, W. L. Hough, T. B. Wilson, A. Pernak, J. Zabielska-Matejuk, A. Fojutowski, K. Kita and R. D. Rogers, Green Chem., 2006, 8, 798 RSC.
- J. Cybulski, A. Wiśniewska, A. Kulig-Adamiak, L. Lewicka, A. Cieniecka-Rosłonkiewicz, K. Kita, A. Fojutowski, J. Nawrot, K. Materna and J. Pernak, Chem.–Eur. J., 2008, 14, 9305 CrossRef CAS PubMed.
- H. Wang, G. Guran and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519 RSC.
- N. Muhammad, Y. A. Elsheikh, M. I. A. Mutalib, A. A. Bazmi, R. A. Khan, H. Khan, S. Rafiq, Z. Man and I. Khan, J. Ind. Eng. Chem., 2015, 21, 1 CrossRef CAS PubMed.
- J. Neumann, S. Steudte, C.-W. Cho, J. Thöming and S. Stolte, Green Chem., 2014, 16, 2174 RSC.
- B. Brycki, M. Waligórska and A. Szulc, J. Hazard. Mater., 2014, 15, 797 CrossRef PubMed.
- J. Ranke, S. Stolte, R. Störmann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107, 2183 CrossRef CAS PubMed.
- N. Ferlin, M. Courty, S. Gatard, M. Spulak, B. Quilty, I. Beadham, M. Ghavre, A. Haiß, K. Kümmerer, N. Gathergood and S. Bouquillon, Tetrahedron, 2013, 69, 6150 CrossRef CAS PubMed.
- A. I. Vogel and B. S. Furniss, Vogel's Textbook of Practical Organic Chemistry, Longman, 4th edn, 1984 Search PubMed.
- J. Nawrot, E. Bloszyk, J. Harmatha, L. Nowotny and B. Drozdz, Acta Entomol. Bohemoslov., 1986, 83, 327 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11710k |
| This journal is © The Royal Society of Chemistry 2015 |