Resonance Rayleigh scattering detection of heparin with concanavalin A
Shuguang Yana,
Yurong Tang*b and
Mengling Yub
aCollege of Energy Resources, Chengdu University of Technology, Chengdu 610059, China
bCollege of Material and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059, China. E-mail: tangyuronga@163.com; Fax: +862884079009; Tel: +862884079009
First published on 1st July 2015
Abstract
Herein we developed a highly sensitive methodology for heparin detection based on a resonance Rayleigh scattering (RRS) spectral assay. Heparin and concanavalin A (conA) densely stacked together and formed nanoparticles or a well-defined brick-and-mortar nanostructure. A strong electrostatic attraction, hydrogen bond and the solid–liquid interface induced RRS enhancement. The result of absorption spectra and TEM testified that the introduction of heparin indeed changed the structure of conA and formed brick-and-mortar nanostructures. The optimum reaction conditions of the method and the reasons for RRS enhancement are also discussed. Combined with an RRS spectral assay, an effective biosensor has been developed for heparin. The bioassay allows sensitive detection of heparin with a detection limit of 2.48 ng mL−1 (3σ), a linear range from 8.28 ng mL−1 to 2.5 μg mL−1. The method was successfully applied to the determination of heparin in heparin sodium injection samples.
Introduction
Heparin is a highly negatively charged sulfated polysaccharide with a heterogeneous mixture of diverse chains of different lengths that consist of repeated copolymers of 1–4 linked iduronic acid and glucosamine residues in a semirandom order.1 This polysaccharide plays an important role in biological systems, and interacts with a wide range of protein targets.2,3 Heparin has been widely used as an anticoagulant during cardiovascular surgery or used to avoid thrombosis.4 However, heparin overdose can induce some adverse effects (such as hemorrhages and thrombocytopenia).5 Beyond the need for simple and ideally real-time continuous measurements of heparin levels in serum during surgery and postoperative therapy periods, there is also a desire for detection methods that can monitor the levels of heparin within infusion solutions to avoid dangerous human errors in dosing, especially for pediatric patients.6 Thus, monitoring the amount of heparin which will be used during the surgery and the anticoagulant therapy is of crucial significance. Traditional clinical procedures for heparin detection rely on the measurements of the activated clotting time or activated partial thromboplastin time.7 These methods are not sufficiently reliable and accurate for clinical settings because of their lack of specificity and potential interference from other factors.8 Thus, many researchers have attempted to develop new methods for the detection of heparin, including fluorimetry,9 phosphorimetry,10 colorimetry,11 light scattering12 and electrochemical methods.13 However, such approaches are either well-selected or cost-efficient but poorly-sensitive and expensive. Furthermore, con A often has poor toxicity profiles.14 The limitations of previous approaches to protamine replacement therapy15 encouraged us to explore an innovative self-assembling approach to multivalent heparin binding.The effect of heparin on protein aggregation would appear from the literature to comprise both promotion and inhibition. These conflicting results could reflect the problem of comparing different proteins with different levels of heparin affinity and different aggregation mechanisms16 which depend on solution pH and ionic strength.17 Protein aggregation could promote formation and interpretation of heparin effects, because protein surface is better conserved and formation of unfolded states is minimized. Protein aggregation is controlled by electrostatics, and the protein surface charge anisotropy regulates protein self-association mechanisms. Electrostatics also control heparin–protein binding. There is a need to explain the possible linkage between electrostatically driven aggregation and heparin–protein binding.
RRS has been known for its sensitivity and simplicity as an analytical technique developed in recent years.18 This technique has been applied successfully to study macromolecules.19,20 RRS is very sensitive to the interaction caused by weak binding forces such as van der Waals interaction force, hydrophobic interaction, and aggregation interaction of biological macromolecules.21 The spectral characteristics and RRS intensity may provide helpful new information to the study of the interaction of biological macromolecules and the molecular recognition.22 Nevertheless, a sensitive, reliable and easily operated detection method for heparin is still expected. Few works, so far, has been reported regarding the foundation of RRS-based protocols to directly detect heparin utilizing protein.
In this paper, the development of novel RRS sensors for the detection of biomolecular specific interactions based on the interaction between heparin and conA was reported. Different combination modes were controlled via the concentration of heparin binding conA in water solutions was firstly assembled. Heparin and conA bind densely together to form nanoparticles and a well-defined brick-and-mortar nanostructure (Scheme 1). A strong electrostatic attraction, hydrogen bond and the solid–liquid interface induce RRS enhancement. To the best of our knowledge, it is the first time that selective binding of conA to heparin has been detected by means of RRS using nanostructured sensors. Therefore, the objective of this study was to develop a facile RRS bioassay for sensitive and selective detection of heparin in biological fluids via a strategy of the target-involved assembly of conA.
 | ||
| Scheme 1 Stepwise macromolecular interactions observed between a conA and heparin, which are accompanied by RRS changes. | ||
Experimental
Materials and reagents
ConA was purchased from Sigma-Aldrich (Shanghai) Trading Co. Ltd. Heparin sodium purchased from Aladdin (Shanghai, China) has 185 USP units per mg. Britton–Robinson buffer solution (BR) was used to control the acidity of the aqueous medium. All the reagents were used for analytical regent grade without further purification and deionized water with conductivity of 18.24 MΩ cm−1 was used in this experiment from a water purification system (ULUPURE, Chengdu, China).Instruments
Transmission electron microscopy (TEM) of heparin–conA complex was carried out on a Tecnai G2 F20 S-TWIN transmission electron microscope at an accelerating voltage of 200 kV (FEI Co., America). Samples were prepared for analysis by evaporating a drop of aqueous product on a lacey carbon copper TEM grid. The UV-vis spectra and RRS spectra were obtained with a U-2910 UV-vis spectrophotometer and an F-7000 fluorescence spectrophotometer (Hitachi Co., Tokyo, Japan).Experimental procedure
22.5 μg mL−1 conA, 150 μL BR (pH = 4.56) and appropriate amounts of heparin were added into a 2 mL colorimetric tube, then diluted with deionized water to the mark and mixed thoroughly with gentle shake. After incubated for 10 min, the RRS and UV-vis spectra of solution were examined.Results and discussion
Nanoparticles assembled by heparin binding conA
To study the interaction between the conA and heparin, a varying concentration of the heparin was added to 22.5 μg mL−1 of conA solution, and the RRS intensity at 294 nm was recorded. As shown in Fig. 1a, the RRS intensity was obviously enhanced with the increasing concentration of heparin, and it reached a maximum of 2.5 μg mL−1 of the heparin in conA solution. The great enhancement of RRS intensity indicated that conA had interacted with heparin to assemble heparin–conA nanoparticles. The process of nanoparticles aggregation is presented in Scheme 1A.However, when the concentration of heparin changed from 2.5 μg mL−1 to 10 μg mL−1, the RRS intensity of the conA solution was obviously reduced with the increasing concentration of heparin (Fig. 1b). We propose that heparin, as a relatively inflexible polymer, organizes conA aggregating along its backbone as a consequence of electrostatic interactions, which interacted with heparin to assemble a well-defined brick-and-mortar nanostructure,23 and then the volume of the scattering nanostructures reduced and the solid–liquid interface disappeared, which produced surface reduced scattering and decreased the scattering intensities. The process of brick-and-mortar aggregation is presented in Scheme 1B. Under the condition of weak acid, heparin carries negative charges, and can bind to the conA through the electrostatic attraction between its negative charges and the positive charges of the amino in conA (Scheme 2). Thus some of them will aggregate each other to form a brick-and-mortar nanostructure. Therefore, it is highly feasible to assemble nanoparticles using electropositive conA and heparin with dense negative charges.
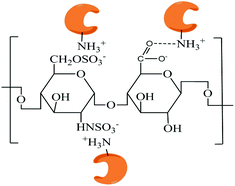 | ||
| Scheme 2 Schematic diagram of the model of the electrostatic attraction and hydrogen bond between conA and heparin. | ||
The interaction between conA and some other mucopolysaccharide (chondroitin sulfate and sodium hyaluronate) were also investigated. The two types of the mucopolysaccharide contain the same backbone and the difference among them lies in their substituent moieties (Fig. 2a and b). As shown in Fig. 2c and d, the RRS intensities appear to be related to the substituent moieties of mucopolysaccharide. Chondroitin sulfate has less negative charges and less sulfonic acid group than heparin, which would lead to instability in the aggregation of nanoparticles. In particular, sodium hyaluronate has no sulfonic acid group, so the binding site for conA is less than other mucopolysaccharide. Therefore, sufficient charges are critical to aggregate nanoparticles when mucopolysaccharide are binding conA. Regarding these two special features, heparin may be the most suitable mucopolysaccharide for assembling brick-and-mortar nanostructure.
The electrostatic attraction between conA and heparin plays a significant role in the aggregation of nanoparticles. In general, the electrostatic attraction is associated with the ionic strength of the solution. Herein, varying concentrations of NaCl were added to conA to control their ionic strength during the binding of nanostructures. As shown in Fig. 3, the RRS intensity decreased gradually with increasing ionic strength of the media, and it attained the minimum at the highest ionic strength. Besides, under the optimal condition, heparin carries negative charges and conA carries positive charges. It can be demonstrated that the electrostatic attraction between the heparin and conA is neutralized after the binding of heparin and conA. Furthermore, the RRS intensity decreased hardly when the excess NaCl was added. The result indicated that in addition electrostatic attraction between the heparin and conA, hydrogen bond may be formed.
TEM analysis of nanoparticles
The RRS intensity is associated with the nanoparticle size of colloid solution. Herein, the size of the nanoparticles was investigated by TEM, and the result is shown in Fig. 4. At lower concentrations of heparin (lower than 2.5 μg mL−1), the diameter of the nanoparticles was close to 300 nm (Fig. 4a). With the increasing number of heparin (2.5 μg mL−1–10 μg mL−1), the structure of the nanoparticle aggregation transformed to brick-and-mortar aggregation (Fig. 4b). | ||
| Fig. 4 (a) TEM image of conA–heparin complex in low concentration of heparin (2.5 μg mL−1). (b) TEM image of conA–heparin complex in high concentration of heparin (10 μg mL−1). | ||
The reasons for the above two opposing size-variation trends may depend on the different properties of large nanoparticles and brick-and-mortar nanostructures. The large nanoparticles were assembled at lower concentration of heparin, where a large number of conA molecules or its micelle structure might be absorbed by a single heparin molecule. The whole large nanoparticle was loose and full of holes due to the electrostatic repulsion of conA molecules. In the case of higher concentration of heparin, because heparin contains many carboxyl groups (–COO−) and the –NH3+ of conA in aqueous solution, it shortens the distance of the neighboring heparin and conA, and compacts the whole nanoparticle through strong hydrogen bonds between the –COO− of heparin and –NH3+ of conA. In this case, the hydrogen bonds would play an important role in maintaining the brick-and-mortar nanostructure.
The reasons for the enhancement and reducing of RRS in low concentration of heparin
Generally, the RRS intensity depends largely on the size of particles under the same experimental conditions. Therefore, it can be indicated that the variation of RRS intensity in solution was probably ascribed to the size alteration of nanoparticles caused by heparin. As for the enhancement mechanism, in theory, RRS enhancing in this system could occur by:![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the peptide chain of proteins bind with –COO− on the surface of a heparin by hydrogen bonds, the original regular and repeating secondary structure of the protein held together by a peptide chain and a hydrogen bond was destroyed and the structure became extended and loose,27,28 which was similar to the denaturing of the protein. This can enhance the scattering.
O) of the peptide chain of proteins bind with –COO− on the surface of a heparin by hydrogen bonds, the original regular and repeating secondary structure of the protein held together by a peptide chain and a hydrogen bond was destroyed and the structure became extended and loose,27,28 which was similar to the denaturing of the protein. This can enhance the scattering.Optimization of general procedure
The influence of buffer solution on the RRS intensity of the system was investigated, such as SCBS, BR and PBS. The results indicated that BR was the best buffer solution of the reaction system. Therefore, in this case, we used the BR of pH 3.29 to 5.72 to investigate the effect of pH on the RRS intensity (Fig. 6a). RRS intensity reached the maximum and remained stable when the pH range was 4–5. The selected pH was 4.56. The most suitable volume of BR buffer solution for the reaction of conA with heparin was 150 μL. (Fig. 6b). The effect of conA concentration on the RRS intensity of the system was investigated. The system had the most suitable sensitivity and the system was stable when the concentration of conA was 22.5 μg mL−1 for the system (Fig. 6c). Therefore, the concentration of conA 22.5 μg mL−1 was suitable. We investigated the factors of reaction time influencing the RRS of the system. The RRS of conA increased quickly in the presence of heparin, and reached stability in 10 min. Therefore, 10 min was chosen for further experiments (Fig. 6d).Sensitivity and selectivity
Under the optimum conditions, the RRS intensities of the system are determined at the maximum scattering wavelength. As indicated in Fig. 1a, the RRS of the conA is sensitive to heparin and linearly increase with the concentration of heparin from 8.28 ng mL−1 to 2.5 μg mL−1 and the limit of detection is 2.48 ng mL−1 (3σ). The linear regression equation is I = 2500C − 146 (where C is the concentration of heparin, μg mL−1). The relative standard deviation (RSD) for eleven replicate detections is 2.07%. It can be seen that this method has a low detection limit and will be a valuable tool for the determination of heparin.Study on the RRS response of the conA to various analytes shows good selectivity of the present assay for heparin. As shown in Fig. 7, only heparin causes a significant increase in relative RRS intensity of conA, while other species have no evident effect on the RRS intensities. The results demonstrate that physiological levels of cation, amino acid and small biomolecules do not interfere with the detection. Specially, large molecules such as lysozyme, papain, BSA and HSA level of 120 μg mL−1, 340 μg mL−1, 120 μg mL−1, 100 μg mL−1 respectively do not interfere with the detection. The charges of conA were maintained and conA still have good solubility in water solution after conA bond glucose. Besides, isoelectric point of lysozyme, papain, BSA and HSA is lower than conA. Therefore, they have positive charges and electrostatic repulsion between conA and lysozyme/papain/BSA/HSA, they do not interfere with the detection.
Application in heparin sodium injection samples
To test the proposed assay, this method was applied to determine heparin in heparin sodium injection samples. A 5 μL portion of heparin sodium injectable was pipetted into a 10 mL calibrated flask and was diluted to the mark with water. A 10 μL portion of this solution of heparin was pipetted into a 20 mL volumetric flask, and then diluted to the mark with water. A 20 μL portion of this solution of heparin was pipetted into a 40 mL volumetric flask, and then diluted to the mark with water. The following procedures are same with the above. The results and recoveries are listed in Table 1. The recoveries were between 97.0% and 101%. Therefore, the method can be applied to the determination of heparin in heparin sodium injection with satisfactory results.| Sample | Found (μg mL−1, n = 5) | Added (μg mL−1) | Total found (μg mL−1, n = 5) | Recovery% |
|---|---|---|---|---|
| 1 | 1.901 | 2 | 3.894 | 97 |
| 2 | 1.941 | 2 | 3.927 | 98 |
| 3 | 2.022 | 2 | 4.047 | 101 |
Conclusions
In summary, we have developed a convenient conA-based detection system for heparin. UV-vis, RRS and TEM indicate that binding to heparin induces nanoscale organization. The large nanoparticles were assembled by lower concentrations of conA and heparin molecules, while the brick-and-mortar nanostructure were constructed under higher concentration of heparin due to electrostatic repulsion among heparin and their hydrogen bonds binding to conA molecules. Such simple materials may be potentially used in biomedical applications. In future work, the ability of this heparin binding protein to intervene in biological processes will be further investigated, as will higher-generation dendritic systems and the potential of these systems to degrade and disaggregate, thus leading to controlled heparin binding and released protein.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21375089 and 21105068). The authors would like to show gratitude for Dr Shanling Wang at Analytical & Testing Center of Sichuan University for her assistance in the TEM analysis. The authors declare no competing financial interest.Notes and references
- D. L. Rabenstein, Nat. Prod. Rep., 2002, 19, 312–331 RSC.
- I. Capila and R. J. Linhardt, Angew. Chem., Int. Ed., 2002, 41, 390–412 CrossRef CAS.
- A. C. Rodrigo, A. Barnard, J. Cooper and D. K. Smith, Angew. Chem., Int. Ed., 2011, 50, 4675–4679 CrossRef CAS PubMed.
- N. Mackman, Triggers, Nature, 2008, 451, 914–917 CrossRef CAS PubMed.
- T. E. Warkentin, M. N. Levine, J. Hirsh, P. Horsewood, R. S. Roberts, M. Gent and J. G. Kelton, N. Engl. J. Med., 1995, 332, 1330–1336 CrossRef CAS PubMed.
- R. Cao and B. X. Li, Chem. Commun., 2011, 47, 2865–2867 RSC.
- P. D. Raymond, M. J. Ray, S. N. Callen and N. A. Marsh, Perfusion, 2003, 18, 269–271 CrossRef CAS.
- M. N. Levine, J. Hirsh, M. Gent, A. G. Turpie, M. Cruickshank, J. Weitz, D. Anderson and M. Johnson, Arch. Intern. Med., 1994, 154, 22–24 CrossRef.
- M. Wang, D. Q. Zhang, G. X. Zhang and D. B. Zhu, Chem. Commun., 2008, 37, 4469–4471 RSC.
- H. Yan and H. F. Wang, Anal. Chem., 2011, 83, 8589–8595 CrossRef CAS PubMed.
- T. Briza, Z. Kejik, I. Cisarova, J. Kralova, P. Martasek and V. Kral, Chem. Commun., 2008, 16, 1901–1903 RSC.
- Y. Ling, Z. F. Gao, Q. Zhou, N. B. Li and H. Q. Luo, Anal. Chem., 2015, 87, 1575–1581 CrossRef CAS PubMed.
- K. L. Gemene and M. E. Meyerhoff, Anal. Chem., 2010, 82, 1612–1615 CrossRef CAS PubMed.
- T. R. Silvers and J. K. Myers, Biochemistry, 2013, 52, 9367–9374 CrossRef CAS PubMed.
- S. M. Bromfield, P. Posocco, C. W. Chan, M. Calderon, S. E. Guimond, J. E. Turnbull, S. Pricl and D. K. Smith, Chem. Sci., 2014, 5, 1484–1492 RSC.
- Y. S. Cho and K. H. Ahn, J. Mater. Chem. B, 2013, 1, 1182–1189 RSC.
- B. B. Minsky, B. Q. Zheng and P. L. Dubin, Langmuir, 2014, 30, 278–287 CrossRef CAS PubMed.
- Y. Shi, H. Q. Luo and N. B. Li, Chem. Commun., 2013, 49, 6209–6211 RSC.
- W. W. Song, N. B. Li and H. Q. Luo, Anal. Biochem., 2012, 422, 1–6 CrossRef CAS PubMed.
- Y. R. Tang, Y. Zhang, Y. Y. Su and Y. Lv, Talanta, 2013, 115, 830–836 CrossRef CAS PubMed.
- L. Kong, Z. F. Liu, X. L. Hu, S. P. Liu and J. D. Meng, J. Lumin., 2013, 137, 186–190 CrossRef CAS PubMed.
- P. P. Li, S. P. Liu, S. G. Yan, X. Q. Fan and Y. Q. He, Colloids Surf., A, 2011, 392, 7–15 CrossRef CAS PubMed.
- A. T. Wright, Z. L. Zhong and E. V. Anslyn, Angew. Chem., Int. Ed., 2005, 44, 5679–5682 CrossRef CAS PubMed.
- S. P. Liu, F. Wang, Z. F. Liu, X. L. Hu, A. R. Yi and H. Duan, Anal. Chim. Acta, 2007, 601, 101–107 CrossRef CAS PubMed.
- Q. Y. Xu, Z. F. Liu, X. L. Hu, L. Kong and S. P. Liu, Anal. Chim. Acta, 2011, 707, 114–120 CrossRef CAS PubMed.
- J. J. Peng, S. P. Liu, L. Wang, Z. W. Liu and Y. Q. He, J. Colloid Interface Sci., 2009, 338, 578–583 CrossRef CAS PubMed.
- S. P. Liu, Z. Yang, Z. F. Liu and L. Kong, Anal. Biochem., 2006, 353, 108–116 CrossRef CAS PubMed.
- J. J. Peng, S. P. Liu, S. G. Yan, X. Q. Fan and Y. Q. He, Colloids Surf., A, 2010, 359, 13–17 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |






