Integrative investigation of Semen Strychni nephrotoxicity and the protective effect of Radix Glycyrrhizae by a UPLC-MS/MS method based cell metabolomics strategy in HEK 293t cell lysates†
Liqiang Gua,
Shujuan Lia,
Ruowen Zhangb,
Yuanyuan Zhanga,
Xiaofan Wanga,
Kexia Zhangc,
Ziying Liua,
Kaishun Bia and
Xiaohui Chen*a
aSchool of Pharmacy, Shenyang Pharmaceutical University, Shenyang 110016, China. E-mail: cxh_syphu@hotmail.com; Fax: +86 2423986259; Tel: +86 2423986259
bStem Cell Institute, Department of Biochemistry and Molecular Genetics, University of Alabama at Birmingham, Birmingham, AL 35294-0024, USA
cSchool of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, Shenyang 110016, China
First published on 24th June 2015
Abstract
Semen Strychni has anti-tumor, analgesic and anti-inflammatory angiogenesis effects, but the clinical use of Semen Strychni is limited by its potential nephrotoxicity. To investigate Semen Strychni nephrotoxicity and the protective effect of Radix Glycyrrhizae, a stable, parallel and repeatable cell metabolomics strategy was developed in this study. After treatment with Semen Strychni, cell morphology was changed, cell viability was decreased and 8 biochemical indexes were altered. Then the developed cell models were successfully applied for a cell metabolomics study. The Semen Strychni group samples were completely separated from the blank group samples in the score plots of PCA and PLS-DA models. Finally, a total of 10 putative biomarkers and 24 related metabolic pathways were screened. Among the 24 metabolic pathways, the taurine and nitrogen metabolic pathways were believed to have the most importance and significance, respectively. Based on the results, the possible mechanisms of Semen Strychni nephrotoxicity might be cellular component disruption, oxidative damage, metabolic waste accumulation and the disturbance of energy and ion transport systems. Meanwhile, the Radix Glycyrrhizae treatment group showed similar behaviors to the blank group in all assays, indicating the great protective effect of Radix Glycyrrhizae against Semen Strychni nephrotoxicity. This cell metabolomics strategy might contribute to investigating the possible nephrotoxic mechanisms of herb medicines and clinical therapies of protective herbs.
Introduction
Nephrotoxicity, one of the most common and serious herbal toxicities, has recently gained much attention around the world. However, standardization and toxicological studies of herb medicines cannot satisfy clinical trials for herbal therapies.1 Hazard identification and risk assessment have long been heavily reliant on the extrapolation of observations in animal models to man, a process which is inherently costly, inaccurate and ethically undesirable.2 Thus, it is very urgent to find an inexpensive and comprehensive strategy to evaluate herbal nephrotoxicity and help clinical therapy.Cell metabolomics, which has lower costs, better repeatability and fewer ethical problems, is developing very fast and becoming a promising nephrotoxicity assessment strategy. Combining cellular models with metabolomics technology, the cell metabolomics strategy (Fig. 1) shows large advantages in nephrotoxicity assessment. Recently, several experiments have established the direct relationships between cells’ metabolic variations and external environment change via cell metabolomics studies,3,4 which showed few of the confounding influences present in animal models or in humans. Furthermore, with the help of metabolomics technology, the knowledge of such relationships may contribute to a better understanding of action mechanisms and pave the way for exploring sensitive metabolites as in vivo markers of therapy response. Moreover, the stability between cell generations, the parallelity of the operating steps and the huge amount of information existing in metabolomics data are also advantages of the cell metabolomics strategy. The HEK 293t cell line, a human embryonic kidney epithelial cell line, was used in this study for its human originated cell type and its wide use in nephrotoxicity assessment.5–7
Based on its advantages, the cell metabolomics strategy might be very appropriate for the investigation of Semen Strychni nephrotoxicity. Semen Strychni is a traditional herb medicine with anti-tumor, analgesic and anti-inflammatory angiogenesis effects.8,9 However, it is limited by its potential toxicity.10,11 In our previous study,12 the nephrotoxicity of Semen Strychni was investigated by determining serum biochemical parameters and observing histopathological slices. Since the mechanism of Semen Strychni nephrotoxicity is still not revealed, the cell metabolomics strategy might play an important role in further studies.
To alleviate the nephrotoxicity caused by Semen Strychni and improve the usage of Semen Strychni in clinics, protective herb therapy is believed to be a reliable approach. The results of our studies showed that Radix Glycyrrhizae could alleviate the renal injury caused by Semen Strychni.12,13 Interactions between Semen Strychni and Radix Glycyrrhizae were also found in previous studies.14,15 Additionally, Radix Glycyrrhizae and its effective components have already demonstrated protective effects in other cell toxicology experiments.16–18 Considering that it is very important to make the usage of Semen Strychni safer, a cell metabolomics study to investigate the protective effect of Radix Glycyrrhizae on Semen Strychni nephrotoxicity is necessary.
Some metabolomics studies have been conducted to reveal the potential nephrotoxicity of herbal medicines by analyzing rat serum or urine samples.19–21 However, cell metabolomics studies of herbal nephrotoxicity, which might further explain the toxic mechanisms by developing direct relationships between kidney cells’ metabolic variation and herb exposure, are limited. Besides, few experiments have been designed to investigate the protective effect of Radix Glycyrrhizae on Semen Strychni nephrotoxicity in kidney cells. In this study, the cell morphology and viability assays were conducted to directly reflect the degeneration of HEK 293t cells. Additionally, the biochemical assays of oxidative stress indexes and nephrotoxicity related enzymes were also used to evaluate the cell function. Then a cell metabolomics study was applied to cluster different groups by principal component analysis (PCA) and partial least squares-discriminant analysis (PLS-DA) models. Finally, the specific biomarkers which were sensitive and reliable were screened and the related metabolic pathways were analyzed to explain the possible mechanism of Semen Strychni nephrotoxicity. The protective effect of Radix Glycyrrhizae was also reflected through these assays by pretreating cells with Radix Glycyrrhizae. The newly developed cell metabolomics strategy could be used not only in investigating the possible nephrotoxic mechanisms of herb medicines, but also in helping therapies of protective herbs.
Experimental
Chemicals and reagents
Semen Strychni and Radix Glycyrrhizae were obtained from Anguo Lengbei Co. (Baoding, China). Aristolochia manshuriensis (positive control herb) was supplied by Shenyang Pharmaceutical University. All the herbs were authenticated by Professor Ying Jia (Pharmacognosy Department, Shenyang Pharmaceutical University). RPMI-1640 medium and fetal bovine serum (FBS) were purchased from Gibco Life Technologies (Melbourne, Australia). Penicillin, streptomycin, dimethyl sulphoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma (St. Louis, MO, USA). All cell culture plastics were from Corning-Costar Co. (NY). The commercial kits used in biochemical assays of catalase (CAT), reduced glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA), nitric oxide (NO), nitric oxide synthase (NOS), glutamine synthetase (GS) and total ATPase were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The reference standards of 5-oxoproline, creatinine, phenylacetylglycine, tryptophan and tyrosine were purchased from Sigma-Aldrich (MO, USA). Acetonitrile, methanol and formic acid (UPLC grade) were purchased from Fisher Scientific (Nanjing, China). Distilled water, prepared with demineralized water, was used for all aqueous solutions throughout the study.Herb preparation
The crude extracts of Semen Strychni, Radix Glycyrrhizae and Aristolochia manshuriensis were extracted by refluxing, then concentrated under a freeze-drying procedure to prepare herbal freeze-dried powders. All the herbal freeze-dried powders were dissolved with RPMI-1640 medium separately and filtrated with 0.22 μm cellulose acetate membrane.Cell culture and treatment
The human embryonic kidney epithelial cell line HEK 293t was originally obtained from Jennio Biotech. Co., Ltd (Guangzhou, China). The cells were cultured with RPMI-1640 medium supplemented with 10% FBS, penicillin (100 U mL−1) and streptomycin (100 μg mL−1), and maintained in exponential growth at 37 °C in a humidified atmosphere under 5% CO2. For treatments, cells were cultured for 10 days prior to experiments. Culture medium was replaced with fresh medium every 48 h.Cells were randomly assigned into four groups (n = 12 per group) as described below. Final concentrations of Semen Strychni, Radix Glycyrrhizae and Aristolochia manshuriensis in all assays were equivalent to 40 mg L−1, 150 mg L−1 and 200 mg L−1 of raw herbs, respectively.
Cell morphology and viability assays
For morphology experiments, cells were seeded on 96-well plates at a density of 1 × 104 per well. After cell treatment as described above, cell morphology was directly observed under an inverted microscope (Olympus).Cell viability was assessed by measuring the capacity of cells to reduce MTT to formazan. After cell morphology was observed, stock MTT solution (10 μL) was added to each well and incubated with the cells for 4 h at 37 °C. Then the supernatant was discarded and 150 μL per well of DMSO was added. After the formazan crystals were completely dissolved on a shaker for 10 min, the optical density of the solution in each well was measured by a microplate reader (Thermo Scientific, Finland) at a wavelength of 490 nm.
Biochemical assays
The HEK 293t cells were seeded in 6-well plates at a density of 1 × 105 per well. After cell treatment as described above, cells were harvested by trypsinization (0.05% trypsin/EDTA solution), then pelleted by centrifugation (1000 rpm, 5 min) and washed with ice-cold phosphate buffered saline (PBS, 0.1 M, pH 7.4). The pellets were resuspended in 200 μL PBS and freeze-thawed twice at −20 °C to obtained the cell lysates. The cell lysates were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (4 °C) for 10 min and the supernatants were collected for biochemical assays.
000 rpm (4 °C) for 10 min and the supernatants were collected for biochemical assays.
As the biochemical indexes are very important in reflecting cell function, six independent oxidative stress assays (CAT, GSH, SOD, MDA, NO, NOS) and two nephrotoxicity related enzyme assays (GS and ATPase) were performed according to the instructions of Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
CAT activity was measured based on the principle that ammonium molybdate could rapidly terminate the action of CAT decomposing H2O2 and react with the residual H2O2 to form a yellow complex (ammonium molybdate method). GSH was detected by reaction with dithiobisnitrobenzoicacid to form a yellow complex. SOD competes with hydroxylamine for O2−˙ and nitrite (the product of hydroxylamine and O2−˙) can be detected by adding a chromogenic reagent (xanthine oxidase method). MDA was detected by the appearance of the conjugated complex of thiobarbituric acid and MDA (thiobarbituric acid method). NO was detected by adding a chromogenic reagent to form an azoic compound (light red). NOS was measured by detecting the colored compound that was created by adding a nucleophilic material. GS can catalyze the reaction of glutamine to form glutamine hydroxyl oxime acid. ATPase activity was measured by detecting inorganic phosphorus, which was decomposed by ATPase from ATP to ADP. The inorganic phosphorus level can reflect the ATPase activity. The levels of these biochemical indexes were expressed as specific activities% (the levels of cells’ specific activities% in BG were set as 100%).
Preparation of cell lysate samples for cell metabolomics analysis
After treatment as described above, cells in different groups were harvested from 6-well plates. About 5 × 105 cells per sample in each group were centrifuged at 1000 rpm for 5 min and washed with PBS twice to get the cell pellets. Then 200 μL ice-cold methanol was added (for metabolism quenching and metabolite extraction) and ultrasonication was performed for 10 min to obtain the cell lysates. After centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (4 °C) for 10 min, the supernatants of the cell lysate samples were collected and stored at −80 °C until analysis.
000 rpm (4 °C) for 10 min, the supernatants of the cell lysate samples were collected and stored at −80 °C until analysis.
Cell metabolomics study by UPLC-MS/MS
Chromatographic analysis was performed in a Waters UPLC-Xevo-TQS system with an electrospray ionization interface. An ACQUITY UPLC HSS T3 (100 mm, 2.1 mm, 1.8 μm) column, which was protected by a HSS T3 VanGuard pre column (5 mm, 2.1 mm, 1.8 μm) was applied in the chromatographic separation at 30 °C. An acetonitrile (solvent B)–water (containing 0.1% formic acid, solvent A) system was used as the mobile phase. The gradient program was shown as follows: 0–2 min, 95–80% A, 2–6 min, 80–70% A, 6–9 min, 70–40% A, 9–10 min, 40–95% A, 10–12 min, 95–95% A. An aliquot of 2 μL of sample solution was injected and the flow rate was 0.2 mL min−1. The optimal conditions of analysis were as follows: source temperature, 150 °C; desolvation gas temperature, 350 °C; cone gas flow, 150 L h−1; desolvation gas flow, 700 L h−1; capillary voltage, 3.0 kV; sampling cone voltage, 30 V; and source offset voltage, 50 V.The fragments of putative biomarkers were acquired with argon (collision gas), and the collision energy varied from 10 to 30 eV. The mass spectrometric data were acquired from 50 to 1000 Da in full scan mode with a 0.3 s scan time and a 0.1 s interscan delay. The total run time was 12 min in both positive and negative ion mode.
Data processing and pattern recognition analysis
The raw obtained mass spectrometric data were pretreated with Micromass MarkerLynx™ Application Version 4.1 (Waters). Processed data with peak selection, alignment and data reduction was acquired. A list of retention time–mass pairs with corresponding intensities for all the detected peaks was also provided. Then, the processed data were exported to SIMCA-P software (version 13.0, Umetrics, Sweden) for further analysis. Two pattern recognition methods, PCA and PLS-DA, were both applied to investigate Semen Strychni nephrotoxicity and the protective effect of Radix Glycyrrhizae. Finally, the sensitive biomarkers were screened by their loading-plot and variable importance in the projection (VIP) values, and the putative metabolic pathways were displayed. SPSS 17.0 software was used to process all the acquired data by one-way analysis of variance (ANOVA) followed by Tukey’s tests.Results and discussion
Dose selection
In this study, a concentration of 40 mg L−1 Semen Strychni was used to develop the cell model of Semen Strychni nephrotoxicity. The dose was chosen by referring to the previous in vivo studies12,22,23 as well as the results of cell morphology and viability assays with a series of concentrations (5, 10, 20, 40, 80 mg L−1). After treatment with concentrations over 40 mg L−1 Semen Strychni for 12 h, cells became round, blurred and easily shedded (Fig. S1†). Meanwhile, cell proliferation was down-regulated and the IC50 value (with three replicates) was near 40 mg L−1. The concentration of 150 mg L−1 Radix Glycyrrhizae was based on the in vivo data24,25 and showed an obvious protective effect. Aristolochia manshuriensis, whose concentration (200 mg L−1) was also determined by IC50 value, was selected to be the positive herb for its specific property of nephrotoxicity.26Cell morphology and viability assays
According to the results of the cell morphology experiment, cells in BG (Fig. 2A) showed a normal appearance of scalene triangle or fusiform shapes with smooth edges. Meanwhile, cells in PCG and SSG (Fig. 2B and C) exhibited obvious injuries, such as clustered cell forms, round shapes and blurred cell edges. Compared with cells in SSG, cells in RGTG (Fig. 2D) showed better appearances with less clustered cell forms, fusiform shapes and smooth cell edges.The Semen Strychni nephrotoxicity and the protective effect of Radix Glycyrrhizae were evaluated by comparing the cell viability of HEK 293t cells in different groups after treatment. Cell growth was significantly inhibited by treatment with Semen Strychni (the cell viability of cells in SSG and PCG was 47.6 ± 5.4% and 55.0 ± 6.2%, respectively). The cell viability of cells in RGTG was 78.7 ± 6.6% (significantly higher than the value of SSG), indicating that pretreatment with Radix Glycyrrhizae might attenuate the nephrotoxicity of Semen Strychni.
The results of cell morphology and viability assays indicated that the Semen Strychni nephrotoxicity cell model was successfully developed and Radix Glycyrrhizae might have protective effects against this nephrotoxicity.
Biochemical assays
After cell morphology and cell viability assays were carried out, biochemical assays of oxidative stress indexes and nephrotoxicity related enzyme levels were performed immediately and carefully in order to make the results reliable. In order to avoid false positive results, parallel experiments (n = 3) for all the assays were also conducted. These assays were conducted to further investigate the cell function after cell treatment.CAT, GSH and SOD are three important indexes in the antioxidant defense system, which can be used to indirectly evaluate cell damage by reflecting the ability of free radical-scavenging.27,28 Meanwhile, MDA (the end product of lipid peroxidation) level can reflect the extent of cell damage due to oxidative stress directly.29 As shown in Fig. 3, when compared with that in BG, the CAT, GSH and SOD activities of cells in SSG were significantly reduced (similar with PCG) by 23%, 74% and 29%, and the MDA levels of cells in SSG were significantly increased (similar to PCG) by 94%. Meanwhile, the pretreatment of Radix Glycyrrhizae resulted in a significant increase of CAT, GSH and SOD levels and a decrease of MDA level in RGTG cells (compared with that in SSG cells).
NO plays an important part in the processes of oxidative stress and apoptosis.30,31 Previous studies reported that a low NO level contributes to the inhibition of cell apoptosis, whereas a higher NO level promotes the destruction of cells.32,33 In this study, relatively low NO levels in different groups were observed (BG, PCG, SSG and RGTG were 35.77 ± 1.24, 8.75 ± 0.80, 7.63 ± 2.07 and 34.33 ± 0.83 mmol L−1, respectively) when compared with the literature.31 Considering that the cell viability of PCG and SSG was significantly lower than that of BG and RGTG, it is probable that the 79% drop in NO level (Fig. 3) in SSG cells indicated a significant difference with that in BG. NOS is a sensitive oxidative stress index which is significantly changed in cells exposed to reactive oxygen species.30,31 In this study, the NOS activity of cells in SSG was significantly decreased (similar with PCG) by 60% when compared with that in BG cells. Fig. 3 also shows that the pretreatment of Radix Glycyrrhizae can significantly increase the levels of NO and NOS in RGTG cells (compared with those in SSG cells).
GS is a very sensitive marker of kidney injury and ATPase plays an important role in material transport, energy transformation and message passing.34,35 In this study, GS and ATPase were measured to reflect the level of cell function. The GS and ATPase levels of cells in SSG were significantly decreased (similar with PCG) by 78% and 25%, respectively, when compared with those in BG. As shown in Fig. 3, the GS and ATPase levels of cells in RGTG were significantly higher than those in SSG cells.
The results of the biochemical assays indicated that Semen Strychni nephrotoxicity might be attributed to several forms of oxidative damage and the inhibition of the activities of nephrotoxicity related enzymes (such as GS and ATPase). The pretreatment of Radix Glycyrrhizae was believed to have a protective effect by alleviating the oxidative damage and improving the enzyme activities.
Cell sample preparation and method validation
Compared with the metabolomics of body fluids, cell metabolomics has stricter requirement in sample processing procedure,36 including metabolism quenching and metabolite extraction. As some metabolites are extremely liable to be metabolized by enzymes, cell metabolism must be immediately quenched upon cell processing. In order to minimize experimental variability in the operation steps, we simplify the sample processing procedure in one step by using methanol as the quenching and extracting solvent. For parallel operations, the same operation procedure was rigorously repeated for all cell lysate samples.The developed method was validated by referring to the literature of method validation strategies in non-targeted metabolomics.37 The typical chromatograms in positive and negative modes are shown in Fig. 4. Precision of injection, within-day stability and sample preparation repeatability were examined prior to the analysis of experimental samples. Quality control (QC) samples were generated by pooling cell lysate samples of different groups and were used during the experiment. The extracted ion chromatographic peaks of eight ions (1.13_114.0, 1.90_182.1, 3.02_277.0, 3.83_437.0, 5.02_194.0, 5.74_726.9, 6.73_148.9 and 8.82_690.9) in positive ion mode and four ions (1.24_124.0, 3.79_213.9, 5.60_391.9 and 8.80_417.8) in negative ion mode were selected for method validation. The selected ions were evenly distributed in the analysis time and in the mass range.
Precision of injection was validated by continuously analyzing six injections of the same QC sample. The results (RSD%) of the retention times and intensities were estimated to be 0.2–0.7% and 6.5–11.5%, respectively. Within-day stability was evaluated by six injections of the same QC sample in 24 h with an interval of 4 h. The results (RSD%) of the retention times and intensities were estimated to be 0.3–0.7% and 7.4–12.0%, respectively. Then six aliquots of a random sample were used to investigate the repeatability of the sample preparation study. The results (RSD%) of the retention times and intensities were estimated to be 0.2–0.6% and 5.9–10.0%, respectively. The method validation results indicated the reliability and repeatability of this method in large scale samples.
Data analysis and biomarker identification
In order to detect subtle metabolomic differences between different groups, a pattern recognition procedure was applied to analyze the cell metabolomics data. Firstly, unsupervised PCA was performed. The PCA score plot showed a good separation between the BG samples and the SSG (close to PCG) samples in both positive and negative ion modes (Fig. 5). Meanwhile, the RGTG samples were clustered closer to the BG samples than the SSG samples. This result indicated that the cell metabolic profile had changed as a result of treating with Semen Strychni and pretreatment with Radix Glycyrrhizae might keep the cell metabolism stable.To further investigate the change in metabolic profile between different groups, supervised PLS-DA was carried out. The R2 values of the PLS-DA model in positive and negative modes were 0.99 and 0.89; and Q2 were 0.96 and 0.63, respectively. These indexes indicated that the developed PLS-DA model showed a good fit and prediction. As shown in Fig. 6A and C, the SSG (close to PCG) samples were clustered separately to the BG samples. And the RGTG samples were near the BG samples.
 | ||
| Fig. 6 PLS-DA score plots (positive ion mode A; negative ion mode C) and loading plots (positive ion mode B; negative ion mode D). | ||
Based on the developed PLS-DA model, loading-plots (Fig. 6B and D) and VIP values were generated to screen the sensitive biomarkers for Semen Strychni nephrotoxicity. In the loading-plot, the y-axis and x-axis denote the contribution of a biomarker to the group difference. The potential biomarkers with the greater distances on the axis were screened. As VIP values >1 were considered to be influential for the group separation in the score plots generated from PLS-DA analysis, we further screened the most sensitive biomarkers with the VIP value. Finally, according to the m/z values and the retention times obtained from the previous screening, the structures of the biomarkers were identified (an example is demonstrated in Fig. S2†) by referring to several online databases (for example, Metlin, HMDB and KEGG) and comparing to our acquired commercial standards. All the detailed information on the 10 screened biomarkers is listed in Table 1.
| Markers | VIP | TR min−1 | m/z (Da) | Formula | Scan mode | Quasi-molecular ion | Putative identification | Content variance | Related pathway | Proposed structure |
|---|---|---|---|---|---|---|---|---|---|---|
| a The biomarkers with reference standards.b p < 0.05 SSG vs. BG. | ||||||||||
| 1 | 1.32 | 0.87 | 203.1 | C10H26N4 | + | [M + H]+ | Spermineb | ↑ | Glutathione metabolism |  |
| 2 | 1.25 | 1.94 | 268.0 | C10H13N5O4 | + | [M + H]+ | Adenosineb | ↓ | Purine metabolism | 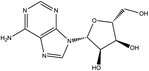 |
| 3 | 1.18 | 3.32 | 205.0 | C11H12N2O2 | + | [M + H]+ | Tryptophana,b | ↓ | Nitrogen metabolism |  |
| 4 | 1.15 | 1.70 | 130.0 | C5H7NO3 | + | [M + H]+ | 5-Oxoprolinea,b | ↑ | Glutathione metabolism |  |
| 5 | 1.11 | 5.02 | 194.0 | C10H11NO3 | + | [M + H]+ | Phenylacetyl-glycinea,b | ↓ | Phenylalanine metabolism |  |
| 6 | 1.10 | 1.13 | 114.0 | C4H7N3O | + | [M + H]+ | Creatininea,b | ↑ | Arginine metabolism |  |
| 7 | 1.07 | 1.90 | 182.1 | C9H11NO3 | + | [M + H]+ | Tyrosinea,b | ↓ | Nitrogen metabolism | 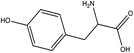 |
| 8 | 1.62 | 5.36 | 145.0 | C5H6O5 | − | [M − H]− | Oxoglutaric acidb | ↓ | TCA cycle |  |
| 9 | 1.60 | 1.24 | 124.0 | C2H7NO3S | − | [M − H]− | Taurineb | ↓ | Taurine metabolism |  |
| 10 | 1.09 | 1.64 | 167.0 | C5H4N4O3 | − | [M − H]− | Uric acidb | ↑ | Purine metabolism |  |
Metabolic pathway analysis and biochemical interpretation
In order to search for possible metabolic pathways that were related to the Semen Strychni nephrotoxicity, the screened biomarkers were imported into MetaboAnalyst. As shown in Fig. 7, taurine metabolism played the most important role (distance on x-axis) and nitrogen metabolism showed the greatest significance (distance on y-axis) among the 24 metabolic pathways involved in nephrotoxicity. The putative biomarkers in this study are also demonstrated on the metabolic pathway net (Fig. 8, referring to KEGG and MetaboAnalyst).Taurine metabolism is a sulfur-containing compounds metabolic pathway in mammals that contributes to many physiological functions. According to a previous study,38 taurine might be regarded as a nephrotoxicity biomarker due to its significantly changed level in renal injury. Taurine can serve as a stabilizer of cell membranes, an antioxidant to oxidative stress and a facilitator in ion transport (such as for sodium, potassium, calcium and magnesium).39 In addition, taurine is the key metabolite in the taurine metabolic pathway, which is finally degraded to several sulfides (Fig. 8). Based on our study results and the literature reports, one of the possible mechanisms of Semen Strychni nephrotoxicity might be explained as the unstable cell membranes and oxidative damage caused by the decreased level of taurine, leading to the disorder of the ion transport system (related to the taurine metabolic pathway).
Nitrogen metabolism is a very important pathway in chronic renal failure.40,41 Tyrosine, tryptophan and taurine are three metabolites which are involved in the nitrogen metabolic pathway.42 As shown in Fig. 8, these three metabolites are degraded to ammonia, then metabolized to nitrogen or nitrile. The literature reports that kidney metabolic disturbance might be caused by accumulated nitrogenous wastes and inorganic ions.41 Hence, the excessive degradation of these nitrogen-containing compounds to nitrogenous wastes might be another reason for Semen Strychni nephrotoxicity.
According to previous reports, some metabolites in the glutathione metabolic pathway are reported to be involved in renal injury.43–45 Glutathione is an antioxidant which prevents the oxidative damage caused by reactive oxygen species to cellular components.43 Fig. 8 shows that spermine and 5-oxoproline are two metabolites in the glutathione metabolic pathway and are closely related to glutathione. When treated with Semen Strychni, the kidney cell components might be affected. Then the balance of the glutathione metabolic pathway could be disrupted, leading to increased levels of spermine and 5-oxoproline. Since phenylacetylglycine is a conjugate of phenylacetic acid and glycine, the disturbance of the glutathione metabolic pathway might also decrease the phenylacetylglycine levels in cells by affecting the biosynthesis and catabolism of glycine.
Creatinine and uric acid are end products in the arginine metabolic pathway and the purine metabolic pathway, respectively. They have been widely used in clinical diagnosis, and increased levels of them are believed to be indicators of nephrotoxicity.46,47 As shown in Fig. 8, uric acid can be catabolized by adenosine through xanthine. Thus, the significantly decreased level of adenosine in SSG might be explained as the excessive transformation to uric acid. The disturbance of the purine metabolic pathway by Semen Strychni might also affect energy metabolism due to the decreased synthesis of ATP from adenosine. The down-regulated oxoglutarate in the TCA cycle after treatment with Semen Strychni indicated the disruption of energy metabolism. It is presumed that a possible mechanism of Semen Strychni nephrotoxicity is the lack of energy in kidney cells.
One of the most difficult problems in the evaluation of herbal nephrotoxicity is the individual difference between different groups (even in the same group). And the repeatability between different batches is another problem to be solved. In the present study, a kidney cell nephrotoxicity model was successfully developed and validated to give a deep insight into the possible mechanisms of Semen Strychni nephrotoxicity with a good stability, parallelity and repeatability. Based on this nephrotoxicity experimental model, a wide disturbance in the cell metabolic pathways was observed (Fig. 7) after treatment with Semen Strychni, indicating the great influence of Semen Strychni on kidney cells.
The possible mechanisms of this nephrotoxicity were concluded as follows: first, Semen Strychni might directly act on the cellular components to disrupt the cell structure; second, Semen Strychni might induce severe oxidative damage which was reflected by the significantly changes of the oxidative stress indexes, leading to the alterations of some endogenous metabolites with low molecular weight; third, since some metabolic pathways were disrupted, the metabolic wastes might accumulate in cells and inhibit cell function; fourth, the influences on the energy transfer and ion transport might be another important mechanism of Semen Strychni nephrotoxicity. The results of this study also showed that the pretreatment of Radix Glycyrrhizae might attenuate the damage caused by Semen Strychni. This cell metabolomics strategy was very promising in the assessment of nephrotoxicity, especially herbal nephrotoxicity, and the pretreatment of protective herbs might have great potential in improving the usage of nephrotoxic herbal medicines in clinics after further investigation. Combined with the cell metabolomics study, further studies are definitely needed to fully reveal the nephrotoxic behavior of Semen Strychni in the future.
Conclusions
In the present study, a cell metabolomics strategy was conducted to investigate Semen Strychni nephrotoxicity and the protective effect of Radix Glycyrrhizae. Significant differences were shown in the cell morphology, cell viability and biochemical assays between the Semen Strychni group and a blank group. The Semen Strychni group samples and blank group samples were completely separated in the score-plots of both PCA and PLS-DA models. A total of 10 putative biomarkers were finally screened and 24 related metabolic pathways were revealed. Cellular component disruption, oxidative damage, metabolic waste accumulation and disturbance of energy and ion transport systems might be explained as the possible mechanisms of Semen Strychni nephrotoxicity. All the results of the Radix Glycyrrhizae treatment group were similar with those of the blank group, indicating the protective effect of Radix Glycyrrhizae. The study demonstrated that the cell metabolomics strategy might be a powerful approach in investigating the mechanisms of herbal nephrotoxicity and the protective effects of herb medicines. This strategy might also provide information on the alteration of numerous metabolic pathways, which would help the study of herbal medicines in the future.Conflict of interest
The authors declare that there are no conflicts of interest.Abbreviations
| CAT | Catalase |
| GS | Glutamine synthetase |
| GSH | Reduced glutathione |
| MDA | Malondialdehyde |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| PCA | Principal component analysis |
| PLS-DA | Partial least squares-discriminant analysis |
| SOD | Superoxide dismutase |
| VIP | Variable importance in the projection |
Acknowledgements
This work was financially supported by Natural Science Foundation of China (No. 81373367).References
- K. Wojcikowski, D. W. Johnson and G. Gobe, J. Lab. Clin. Med., 2006, 147, 160–166 CrossRef PubMed.
- J. K. Ellis, T. J. Athersuch, R. Cavill, R. Radford, C. Slattery, P. Jennings, T. McMorrow, M. P. Ryan, T. M. Ebbels and H. C. Keun, Mol. BioSyst., 2011, 7, 247–257 RSC.
- D. A. Ouattara, J. M. Prot, A. Bunescu, M. E. Dumas, B. Elena-Herrmann, E. Leclerc and C. Brochot, Mol. BioSyst., 2012, 8, 1908–1920 RSC.
- Y. Noguchi, J. D. Young, J. O. Aleman, M. E. Hansen, J. K. Kelleher and G. Stephanopoulos, Mol. BioSyst., 2011, 7, 1409–1419 RSC.
- M. I. Waly, B. H. Ali and A. Nemmar, Toxicol. In Vitro, 2013, 27, 2299–2304 CrossRef CAS PubMed.
- X. L. Shen, Y. Zhang, W. Xu, R. Liang, J. Zheng, Y. Luo, Y. Wang and K. Huang, J. Proteomics, 2013, 78, 398–415 CrossRef CAS PubMed.
- M. I. Waly, B. H. Ali, I. Al-Lawati and A. Nemmar, J. Appl. Toxicol., 2013, 33, 626–630 CrossRef CAS PubMed.
- S. Saraswati, A. A. Alhaider and S. S. Agrawal, Chem.–Biol. Interact., 2013, 206, 214–221 CrossRef CAS PubMed.
- W. Yin, T. S. Wang, F. Z. Yin and B. C. Cai, J. Ethnopharmacol., 2003, 88, 205–214 CrossRef CAS.
- B. S. Naik and M. Chakrapani, Malays. J. Pathol., 2009, 31, 67–69 Search PubMed.
- A. Lages, J. Pinho, R. Alves, C. Capela, E. Lourenço and L. Lencastre, Journal of Medical Cases, 2013, 4, 385–388 Search PubMed.
- L. Q. Gu, X. F. Wang, Z. Z. Liu, P. Ju, L. H. Zhang, Y. Y. Zhang, B. J. Ma, K. S. Bi and X. H. Chen, Food Chem. Toxicol., 2014, 68, 226–233 CrossRef CAS PubMed.
- L. Q. Gu, X. F. Wang, Y. Y. Zhang, Y. Jiang, H. Lu, K. S. Bi and X. H. Chen, J. Sep. Sci., 2014, 37, 1058–1066 CrossRef CAS PubMed.
- S. Y. Zhang, N. N. Song, F. Yu, J. Gao, Y. Zeng and C. X. Liu, Asian J. Phar. Pharmacokinet., 2009, 9, 277–283 Search PubMed.
- L. Liu, J. Xiao, Z. Peng, W. Wu, P. Du and Y. Chen, Chin. Herb. Med., 2012, 4, 118–125 CAS.
- L. Teng, C. Kou, C. Lu, J. Xu, J. Xie, J. Lu, Y. Liu, Z. Wang and D. Wang, Int. J. Mol. Med., 2014, 34, 742–748 CAS.
- K. S. Suh, S. Y. Rhee, Y. S. Kim and E. M. Choi, Food Funct., 2014, 5, 1432–1440 CAS.
- Q. Shi, Y. Hou, Y. Yang and G. Bai, Biol. Pharm. Bull., 2011, 34, 609–617 CAS.
- P. Guo, D. Wei, J. Wang, G. Dong, Q. Zhang, M. Yang and L. Kong, RSC Adv., 2015, 5, 27018 RSC.
- Y. Tan, J. Ko, X. Liu, C. Lu, J. Li, C. Xiao, L. Li, X. Niu, M. Jiang, X. He, H. Zhao, Z. Zhang, Z. Bian, Z. Yang, G. Zhang, W. Zhang and A. Lu, Mol. BioSyst., 2014, 10, 2305–2316 RSC.
- C. Ma, K. S. Bi, M. Zhang, D. Su, X. Fan, W. Ji, C. Wang and X. H. Chen, J. Pharm. Biomed. Anal., 2010, 53, 559–566 CrossRef CAS PubMed.
- Y. Y. Xu, D. Y. Si and C. X. Liu, J. Pharm. Biomed. Anal., 2009, 49, 487–491 CrossRef CAS PubMed.
- Y. W. Liu, R. H. Zhu, H. D. Li, M. Yan and Y. Q. Lei, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 2714–2719 CrossRef CAS PubMed.
- Y. Yan, C. Z. Chai, D. W. Wang, J. Wu, H. H. Xiao, L. X. Huo, D. N. Zhu and B. Y. Yu, J. Pharm. Biomed. Anal., 2014, 95, 76–84 CrossRef CAS PubMed.
- B. L. Xu, P. Y. Li and G. J. Zhang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2015, 988, 33–44 CrossRef CAS PubMed.
- S. L. Hu, H. Q. Zhang, K. Chan and Q. X. Mei, Toxicology, 2004, 198, 195–201 CrossRef CAS PubMed.
- N. Jia, T. Li, X. Diao and B. Kong, J. Funct. Foods, 2014, 11, 142–151 CrossRef CAS PubMed.
- B. Zhang, X. Peng, G. Li, Y. Xu, X. Xia and Q. Wang, Toxicon, 2015, 94, 1–7 CrossRef CAS PubMed.
- J. Jiang, S. Yu, Z. Jiang, C. Liang, W. Yu, J. Li, X. Du, H. Wang, X. Gao and X. Wang, Oxid. Med. Cell. Longevity, 2014, 2014, 310504 Search PubMed.
- L. Zhai, P. Zhang, R. Y. Sun, X. Y. Liu, W. G. Liu and X. L. Guo, Pharmacol. Rep., 2011, 63, 1469–1480 CrossRef CAS.
- X. F. Zhong, G. D. Huang, T. Luo, Z. Y. Deng and J. N. Hu, Mol. Med. Rep., 2012, 5, 1261–1266 CAS.
- B. M. Choi, H. O. Pae, S. I. Jang, Y. M. Kim and H. T. Chung, J. Biochem. Mol. Biol., 2002, 35, 116–126 CrossRef CAS.
- Y. H. Shen, X. L. Wang and D. E. Wilcken, FEBS Lett., 1998, 433, 125–131 CrossRef CAS.
- E. Zanetti, A. Chiusolo, R. Defazio, A. Casartelli, E. Cappelletti, N. Bocchini, F. Chiara, P. Cristofori and A. Trevisan, J. Appl. Toxicol., 2010, 30, 142–150 CAS.
- Y. R. Zhang and Z. Y. Yuan, Clin. Exp. Pharmacol. Physiol., 2010, 37, 613–618 CrossRef CAS PubMed.
- Z. León, J. C. García-Cañaveras, M. T. Donato and A. Lahoz, Electrophoresis, 2013, 34, 2762–2775 Search PubMed.
- S. Naz, M. Vallejo, A. García and C. Barbas, J. Chromatogr. A, 2014, 1353, 99–105 CrossRef CAS PubMed.
- K. B. Kim, S. Y. Um, M. W. Chung, S. C. Jung, J. S. Oh, S. H. Kim, H. S. Na, B. M. Lee and K. H. Choi, Toxicol. Appl. Pharmacol., 2010, 249, 114–126 CrossRef CAS PubMed.
- S. Roysommuti and J. M. Wyss, Amino Acids, 2014, 46, 57–72 CrossRef CAS PubMed.
- J. D. Kopple, F. J. Monteon and J. K. Shaib, Kidney Int., 1986, 29, 734–742 CrossRef CAS PubMed.
- B. J. Maroni, Miner. Electrolyte Metab., 1998, 24, 34–40 CrossRef CAS PubMed.
- J. Márquez, F. Sánchez-Jiménez, M. A. Medina, A. R. Quesada and I. Núñez de Castro, Arch. Biochem. Biophys., 1989, 268, 667–675 CrossRef.
- N. Jiang, L. Lu, T. Wang, L. Zhang, W. Xin and F. Fu, Toxicol. Mech. Methods, 2010, 20, 69–74 CrossRef CAS PubMed.
- K. Zahedi, S. Barone, Y. Wang, T. Murray-Stewart, P. Roy-Chaudhury, R. D. Smith, R. A. Casero Jr and M. Soleimani, PLoS One, 2014, 9, e110161 Search PubMed.
- C. L. Foot, J. F. Fraser and D. V. Mullany, Nephrol., Dial., Transplant., 2005, 20, 2836–2838 CrossRef PubMed.
- A. S. Levey, J. Coresh, T. Greene, L. A. Stevens, Y. L. Zhang, S. Hendriksen, J. W. Kusek and F. Van Lente, Ann. Intern. Med., 2006, 15, 247–254 CrossRef.
- I. Ohno, T. Hosoya, H. Gomi, K. Ichida, H. Okabe and M. Hikita, Nephron, 2001, 87, 333–339 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra07708g |
| This journal is © The Royal Society of Chemistry 2015 |







