UV photocatalytic activity of Au@ZnO core–shell nanostructure with enhanced UV emission†
Abstract
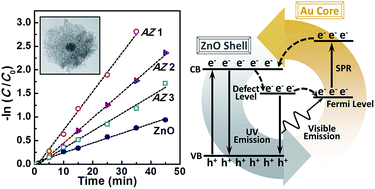
Zinc oxide (ZnO)–gold (Au) nanostructures have been extensively studied for their photocatalytic performance. However, there are still certain open questions remaining, such as the photocatalysis of Au@ZnO core–shell nanostructures under ultraviolet (UV) light and the correlation between their photocatalytic and photoluminescence properties. In this study, we have produced a series of Au@ZnO nanostructures with the Au core surrounded by small ZnO nanocrystallines. The strong visible emission in the green light region of the small ZnO nanocrystallines enables an internal bidirectional electron transfer between Au and ZnO, including the transfer from the defect level of ZnO to the Fermi level of Au, and the excited electrons in Au through surface plasmon resonance absorption of the ZnO green emission to the conduction band of ZnO. The electron circulation induces increased UV and quenched visible emissions. We demonstrate that such Au@ZnO possesses remarkably enhanced UV photocatalytic capability. This study may have provided a deeper understanding of the mechanisms and property optimization of metal–semiconductor nanostructures for photocatalytic applications.

 Please wait while we load your content...
Please wait while we load your content...