Nanotopological-tailored calcium phosphate cements for the odontogenic stimulation of human dental pulp stem cells through integrin signaling
So-Youn Lee†
a,
Hyung-Mun Yun†a,
Roman A. Perezbcd,
Sara Gallinettid,
Maria-Pau Ginebrad,
Seong-Jun Choibc,
Eun-Cheol Kim*a and
Hae-Won Kim*bce
aDepartment of Oral and Maxillofacial Pathology and Research Center for Tooth and Periodontal Regeneration (MRC), School of Dentistry, Kyung Hee University, 1 Heogi-dong, Dongdaemun-gu, Seoul, 130-701, Republic of Korea. E-mail: eckim@khu.ac.kr; Fax: +82-2-960-1457; Tel: +82-2-961-0746
bInstitute of Tissue Regeneration Engineering (ITREN), Dankook University, Cheonan 330-714, Republic of Korea. E-mail: kimhw@dku.edu; Fax: +82 41 550 3085; Tel: +82 41 550 3081
cDepartment of Nanobiomedical Science & BK21 PLUS NBM Global Research Center for Regenerative Medicine, Dankook University, Cheonan, Republic of Korea
dBiomaterials, Biomechanics and Tissue Engineering Group, Department of Materials Science and Metallurgy, Technical University of Catalonia (UPC), Avda. Diagonal 647, E-08028 Barcelona, Spain
eDepartment of Biomaterials Science, College of Dentistry, Dankook University, Cheonan, Republic of Korea
First published on 16th July 2015
Abstract
Calcium phosphate cements (CPCs) are a unique class of inorganic injectables attractive for the repair and regeneration of hard tissues. Tailoring the crystallite properties of CPC, particularly to represent nanotopological features, is favorable for stimulating biological reactions. Nanotopological tailoring has recently been achieved on CPCs by simply modulating the sizes of the initial particles. Herein, we aim to investigate the effects of nanotopological-tailored CPCs on the odontogenic differentiation of stem cells derived from human dental pulp (HDPSCs) as well as on their implicated signal pathways. The initial adhesion of the cells was substantially higher on nano-CPCs than on micro-CPCs. A series of indications of odontogenesis, including alkaline phosphatase activity and gene expressions (dentin matrix protein-1, dentin sialophosphoprotein, osteocalcin, ostepontin, and bone sialoprotein) were significantly stimulated on the nano-CPC in comparison to the micro-CPC. Furthermore, the integrin downstream pathways of the cells, including FAK, paxillin, Akt, MAPK, and NF-κB, were highly activated on the nano-CPC with respect to those on the micro-CPCs. Collectively, the nanotopological CPCs significantly enhance the odontogenic differentiation of HDPSCs when compared to conventional micro-CPCs through the integrin-associated signaling pathways, which implies that the nanotopological CPCs may be more potent in the repair and regeneration of dentin–pulp complex tissues.
1. Introduction
The regeneration of dental pulp-dentin tissues ultimately targets preserving teeth via endodontic approaches. Dental pulp tissue engineering requires a triad of dental pulp stem cells (DPSCs), morphogens (including growth factors and cytokines), and scaffolds.1 The successful regeneration of dental pulp requires the DPSCs to differentiate adequately, secreting extracellular matrix molecules in dental-pulp tissues such as dentin sialoprotein (DSP), dentin phosphoprotein (DPP), dentin matrix protein (DMP-1), osteocalcin (OCN), osteopontin (OPN), and bone sialoprotein (BSP).2,3Calcium phosphate cements (CPCs) are well-known bioactive synthetic bone graft materials.4 They can form in an aqueous environment to hydroxyapatite, a mineral phase of bone and dentin.5,6 CPCs thus exhibit excellent biocompatibility, osteoconductivity, and sealing ability, and provide new options for root canal treatments as a filler/sealer.7,8 In addition, the self-setting and the moderate compressive strength even in loading conditions suggests that CPCs are superior to pure calcium hydroxides; which means that CPCs may have applications in inducing reparative dentin formation in pulp capping or as a lining material.9 Previously, we reported that CPCs and the composites with biopolymers facilitated growth and differentiation of human DPSCs.10 In addition, we demonstrated that newly developed calcium phosphate-based sealers (CAPSEAL I and II) showed higher cell viability and lower inflammatory mediation in periodontal ligament cells when compared to other sealers, showing the potential of these new materials to promote bone regeneration.11 However, the slow degradation rate with limited hard tissue ingrowth has also been suggested that needs improvement in those cementation materials.12,13
The properties of the CPCs, including their calcium deficient hydroxyapatite (CDHA) crystalline microstructure, porosity and pore size and specific surface area, are dependent on the characteristics of the initial CPC powders and can determine the adsorption rate and the release kinetics of proteins.14 Previous studies have shown that the variation in the particle size (micro or nano) of the powders allows the CPCs to develop highly distinct microstructures, either plate-like or needle-like structure.15,16 The compressive strength of the CPCs after hardening increases as the surface area increases, which suggests that a stronger CPC can be derived from the smaller particle size of the raw materials.17 Therefore, the particle size is the key factor that determines the final properties of the CPCs, especially by affecting the kinetics of the chemical reaction and the mechanical consolidation of the cements. The reduction in the particle size has been reported to produce a substantial decrease in the setting time and to accelerate the hardening of the CPCs.18 Furthermore, the different topography of the substrates has been shown to significantly affect the behavior of osteoblast-like cells, showing a slight decrease in the proliferation rate but with substantially improved differentiation activity.15,19
However, the role that the different topology of CPCs plays particularly in the odontogenic differentiation, and its relevant signal transduction mechanisms remain unclear. To elucidate this, here we developed two different-topological CPCs, namely nano-sized and micro-sized CPC, and the effects of the different topology on the odontogenic potential and the underlying signal mechanisms in human dental pulp stem cells (HDPSCs) were investigated.
2. Materials and methods
2.1. Preparation of nano- and micro-sized CPC powders and settings
The CPC powder phase was composed of α-TCP, which was obtained from the mixture of calcium hydrogen phosphate (CaHPO4; Sigma-Aldrich C7263) and calcium carbonate (CaCO3; Sigma-Aldrich C4830), after heat-treatment at 1400 °C for 15 h (Hobersal CNR-58), followed by quenching. The α-TCP was milled in a Pulverisette 6 planetary ball mill (Fritsch GmbB) following two different protocols, as previously described,19 to obtain for the starting powders of the CPC in the form of nano-CPC (CPC-N) and micro-CPC (CPC-M) samples, which have nano-sized and micro-sized crystalline morphology, respectively. The particle size distribution of the powders was analyzed by laser diffraction (LS 13 320 Beckman Coulter), dispersing the powders in ethanol in an ultrasonic bath to avoid aggregation. 2 wt% precipitated hydroxyapatite (PHA; Alco ref. 1.02143) was added as a seed. The liquid phase was composed of an accelerant solution (2.5% Na2HPO4), and the powder and the liquid were mixed at an L/P ratio of 0.65 (ml g−1). The mixture was introduced into Teflon disk moulds with diameters of 15 mm and a height of 2 mm. The CPC disks were then left to set in a Ringers solution at 37 °C for 7 days to obtain a complete transformation of the α-TCP into CDHA. The samples were finally sterilized by ethanol immersion for 1 hour. The samples were then placed in 24 well plates and were washed 3 times with PBS to remove any remaining ethanol. The samples were then left overnight in the presence of culture media in order to completely wet the pores as well as the surface of the CPCs.| Genes | Primer sequence (5′–3′) | Annealing temp (°C) | Cycle number | Product size (bp) |
|---|---|---|---|---|
| DSPP | F: 5′-AGAAGGACCTGGCCAAAAAT-3′ | 60 | 35 | 280 |
| R: 5′-TCTCCTCGGCTACTGCTGTT-3′ | ||||
| DMP-1 | F: 5′-GATCAGCATCCTGCTCATGTT-3′ | 55 | 35 | 125 |
| R: 5′-AGCCAAATGACCCTTCCATTC-3′ | ||||
| OCN | F: 5′-GTGCAGCCTTTGTGTCCAAGCAGGA-3′ | 60 | 30 | 244 |
| R: 5′-CCGTAGAAGCGCCGATAGGCC-3′ | ||||
| OPN | F: 5′-CCCACAGACCCTTCCAAGTA-3′ | 60 | 35 | 196 |
| R: 5′-GGGGACAACTGGAGTGAAAA-3′ | ||||
| BSP | F: 5′-TGGAGATGACAGTTCAGAAG-3′ | 52 | 35 | 333 |
| R: 5′-GTACTGGTGCCGTTTATGC-3′ | ||||
| Integrinα1 | F: 5′-TGCTGCTGGCTCCTCACTGTTGT-3′ | 61 | 32 | 135 |
| R: 5′-TAGTCTGGCGGCCACCTCTCTG-3′ | ||||
| Integrinα2 | F: 5′-TTTCCCTGCTCTCACCGGGC-3′ | 61 | 32 | 412 |
| R: 5′-ACCGGGGGACCGTAGTTGCG-3′ | ||||
| Integrinβ1 | F: 5′-GACGCCGCGCGGAAAAGATG-3′ | 61 | 35 | 159 |
| R: 5′-ACCACCCACAATTTGGCCCTGC-3′ | ||||
| β-Actin | F: 5′-CATGGATGATGATATCGCCGCG-3′ | 60 | 35 | 371 |
| R: 5′-ACATGATCTGGGTCATCTTCTCG-3′ |
2.2. Characterization of CPCs
Mercury intrusion porosimetry (MIP; Autopore IV Micromeritics) was carried in order to examine the porosity content (open porosity) and to determine the pore size distribution within the hardened CPC. The specific surface area of the CPC was measured via N2 adsorption according to the BET method (ASAP 2020; Micromeritics). The crystalline phases were assessed via X-ray diffraction (XRD; Philips MRD). Ni-filtered Cu Kα radiation was used and the step-scanning was performed with an integration time of 50 s at intervals of 0.017° 2θ. The peaks were indexed by means of the JCPDS cards 29-359 for α-TCP and 9-432 for HA. The relative amounts of the different phases that were present in the samples after 7 days in water were estimated on the basis of the peak intensity variation by means of the external standard method, with a pure α-TCP as the external standard.2.3. Cell culture
The immortalized HDPSCs were transfected with a telomerase catalytic subunit of a human telomerase reverse transcriptase (hTERT),20 kindly provided by Professor Takashi Takata (Hiroshima University, Japan). The cells were cultured in α-MEM supplemented with 10% FBS, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin in a humidified atmosphere of 5% CO2 at 37 °C. In order to induce differentiation, the cells were cultured in an osteogenic medium (OM; 50 μg ml−1 ascorbic acid, 10 mM β-glycerophosphate, and 100 nM dexamethasone) as previously described.21–24 The culture medium was exchanged every 2 days, and upon confluence, the cells were detached with a minimum amount of trypsin–EDTA (Gibco) that was inactivated with FBS after 5 min. The cells were then either re-cultured or used for further experiments.A direct cell culture method was performed on the CPC to assess the effect that micro or nano CPCs had in the presence of odontogenic media. 3 × 105 HDPSCs were seeded on each sample. As a control, the same number of cells was cultured at the bottom of the 24 well plates which was maintained in normal proliferation media as well as osteogenic media.
2.4. MTT assay
The cell viability was determined by a 3-(4,5-dimethylthiazolyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT). The MTT is based on a WST-1 reaction that produces a purple formazan dye in an amount that is directly related to the number of viable cells. After 3, 7 and 14 days, the medium was removed and replaced with 200 μl of serum-free medium followed by an addition of 20 μl of MTT solution. The reagent was left to react for 4 h and then the reactant was read at an absorbance of 450 nm. A minimum of four replicates were assayed for each condition.2.5. Cell morphology by fluorescence microscopy
The morphology of the cells was further observed via fluorescence microscopy (Cell Voyager, Yokogawa, Japan). After 60 min, the cells were fixed with 4% paraformaldehyde solution and were incubated first with mouse monoclonal anti-human p65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and then with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Invitrogen life technology, Grand Island, NY). The cells were then counter-stained for nuclei with 10 μg ml−1 of propidium iodide.2.6. Cell adhesion assay
The cells were seeded in nano-CPC or micro-CPC and were then cultured for 1, 2, 4, and 8 h. The adhesion was determined by using a CytoSelect Cell Adhesion Assay Kit (Cell Biolabs, San Diego, CA). Briefly, after 30 min of incubation at 37 °C, the adherent cells were fixed, stained and colorimetrically quantified in a plate reader at 560 nm. The percentage was calculated by comparing the absorbance of each material to that of negative control.2.7. ALP activity assay
As a marker of early osteoblast differentiation, alkaline phosphatase (ALP) activity was assessed in 24-well plates after 14 days of incubation in media supplemented with OM and CPC. The cells were then washed with PBS, and then the cell layers were scraped into a solution containing 20 mM Tris–HCl, pH 8.0, and 150 mM NaCl, 1% Triton X-100, 0.02% NaN3, 1 μg ml−1 aprotinin. The lysates were homogenized. Then, the ALP activity was assayed via spectrophotometric measurements (410 nm, Beckman Coulter, Fullerton, CA) of p-nitrophenol release at 37 °C. In order to normalize protein expression to total cellular protein, a fraction of the lysate solution was used in a Bradford protein assay (n = 4).2.8. Semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
The total RNA of the pulp cells was extracted using a TRIzol reagent (Life Technologies, Gaithersburg, MD) according to the manufacturer's instructions, and 1 μg RNA was reverse-transcribed for the first-strand cDNA synthesis (Gibco BRL, Rockville, MD). The cDNA was amplified in 20 μl with 2.5 mM of magnesium dichloride, 1.25 units of Ex Taq polymerase (Bioneer, Daejeon, Korea) and 1 μM of specific primers. Amplification took place for 30 cycles in a DNA thermal cycler, and the primer sequences for the differentiation markers are summarized in Table 1. The PCR products were resolved on a 1.5% agarose gel and were stained with ethidium bromide.2.9. Western blot analysis
The cells (3 × 105) were cultured to 70% confluence in a 6-well plate and were treated for the indicated times under OS or CPC. The cells from each set of the experiments were harvested and were washed twice in cold Tris-buffered saline. The cells were solubilized in ice-cold 1% Triton X-100 lysis buffer. After 30 min on ice, the lysates were clarified by centrifugation. The proteins (20 μg) were resolved via SDS-PAGE (10% acrylamide) and were transferred to nitrocellulose membranes, and were probed with specific Abs (diluted 1/1000), followed by incubation with secondary horseradish peroxidase-conjugated Ab (1/100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). The proteins were detected by enhanced chemiluminescence system (Amersham, Piscataway, NJ) according to the manufacturer's instructions and exposed to X-ray.
000). The proteins were detected by enhanced chemiluminescence system (Amersham, Piscataway, NJ) according to the manufacturer's instructions and exposed to X-ray.
2.10. Statistical analysis
The differences among the groups were analyzed using a one-way analysis of variance combined with the Bonferroni test. All of the values were expressed as mean ± standard deviation, and the differences were considered significant at p < 0.05.3. Results
3.1. Initial particles used for the nano- and micro-CPCs
Table 2 summarizes the characteristics of the initial particles used for the nano- and micro-CPCs. While the initial particle size for the micro-CPCs was 5.84 μm that for the nano-CPCs was 2.44 μm. Furthermore, the specific surface area of the micro-CPC particles was 1.23 m2 g−1, and that of the nano-CPC particles was 3.05 m2 g−1.| For micro-CPC | For nano-CPC | |
|---|---|---|
| Particle size (μm) | 5.841 ± 0.572 | 2.444 ± 0.048 |
| Specific surface area (m2 g−1) | 1.228 ± 0.008 | 3.053 ± 0.019 |
3.2. Physico-chemical properties of nano- and micro-CPCs
When the CPC particles were hardened with liquid, the phase of the CPCs was examined by XRD, as shown in Fig. 1. A complete transformation of the initial α-TCP into HA was observed during the 7 days of reaction in an aqueous solution. The crystalline morphologies of the hardened nano- and micro-CPCs were observed by SEM after a complete transformation into HA, as shown in Fig. 2. While a large-sized plate-like morphology could be seen for the micro-CPCs, a much smaller-sized (far less than 1 μm) needle-like shape was obtained for the nano-CPCs, revealing the significant influence of the initial particle size had on the crystalline nano-/micro-morphology of cements.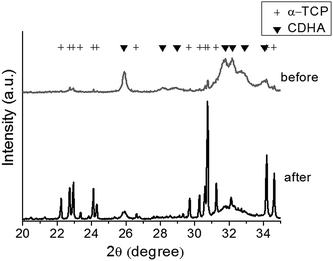 | ||
| Fig. 1 XRD patterns of the CPC powders, showing initial alpha-TCP phase that transformed completely to calcium-deficient hydroxyapatite (CDHA) after immersion for 7 days. | ||
Fig. 3 shows the pore size distribution of the micro- and nano-CPCs. The micro-CPCs presented a higher pore population in the micrometric range (1.20 μm) while the nano-CPCs tended to have fewer micrometric pores and higher nanometric pores (lower than 1 μm). Despite the considerable differences in the pore size, the porosities were shown to be identical with a value of 54%. The specific surface area also showed a considerable difference, with a value of 18.98 m2 g−1 for the micro-CPCs and a value of 39.05 m2 g−1 for the nano-CPCs.
 | ||
| Fig. 3 Characterization of pore size distribution of hardened CPC samples, showing nano-sized pores in the nano-CPC and micro-sized pores in the micro-CPC. | ||
3.3. Effects on the cell adhesion and proliferation
The HDPSCs were cultured either with control medium or with odontogenic medium (OM) on the culture dish or on the CPCs. The cell adhesion rate was measured for up to 8 h (Fig. 4A). Cell adhesion appeared to be higher on the CPC-N than on the CPC-M at all culture periods, but a statistical significant difference was only noticed at 4 and 8 h. The cells adhered initially actively spread and grew on all sample groups. The cell viability was then evaluated up to 14 days, by means of an MTT assay. When the cells were cultured without OM, both CPC groups showed slightly lower cell viability primarily during the initial period. When cultured with OM, the cell proliferation significantly improved, which was also clearly shown in the CPC-cultured cells. The results showed that both CPC groups substantially supported the growth of the HDPSCs for up to 14 days, with fairly comparable levels to those of culture dish culture.3.4. Effects on odontogenic differentiation
The effects of the nano-/micro-topologies of CPCs on the odontogenic differentiation of HDPSCs were assessed by the ALP activity and the mRNA levels of odontogenic genes. Fig. 5A shows the ALP activity of the HDPSCs after culture for 7 and 14 days. Without OM, both CPC groups showed a substantial increase in the ALP activity during both periods. When cultured under the OM conditions, the increase in the ALP activity was more considerable. Of note, the CPC-N showed significantly higher levels than the CPC-M for both culture periods. The gene expression related to odontogenesis (e.g. DMP-1, DSPP, OCN, OPM, and BSP) was observed at 7 and 14 days of culture (Fig. 5B). The RT-PCR band intensity was much higher under the OM conditions (vs. without OM) for the groups. Furthermore, the band intensity of the CPC-N was much higher than that of the CPC-M, particularly with OM at both periods.3.5. Signal transduction pathways
In order to determine the signaling pathways involved in the odontogenesis of HDPSCs by the topological-tailored CPCs, the integrins and the intracellular adhesion molecules in the downstream pathways were analyzed in terms of gene and protein expressions, respectively. The mRNA levels of the integrin subsets, including α1, α2, and β1 were upregulated via nano- and micro-CPC in a time-dependent manner relative to the CPC-free culture dish (Fig. 6A). Furthermore, there was a substantial difference between CPC-N and CPC-M. The integrin downstream signaling proteins in phosphorylated form, including p-FAK, p-paxillin, and p-Akt, were further analyzed by Western blot (Fig. 6B). Results showed considerably enhanced values, particularly p-FAK and p-Akt, in the CPC-N than in the CPC-M.Since the integrin downstream signal pathway is involved in the activation of the mitogen-activated protein kinase (MAPK) and of the nuclear factor-κB (NF-κB),25 we next examined whether the nano- or the micro-CPC treatment affected the protein expressions of the MAPK and NF-κB. The phosphorylation of p38, c-Jun N-terminal kinase (JNK) and the extracellular signal-related kinase (ERK) was higher on CPC-N than on CPC-M (Fig. 7A). As shown in Fig. 7B, the CPC-N induced substantial level of phosphorylation and degradation in the IκBα as well as in the nuclear translocation of p65 (NF-κB). In a similar manner, the confocal images showed that NF-kB p65 in the nucleus markedly increased by CPC-N than by CPC-M (Fig. 7C).
4. Discussion
In this study, we examined whether the crystalline size of the CPCs had any beneficial effects on the odontogenic potential of HDPSCs. Because HDPSCs are potent cell source for the applications in endodontic regeneration, they are considered to provide a promising assessment tool for the odontogenic differentiation effects of the biomaterials developed.26,27Our study indicated that the nano-/micro-topological parameter of the CPCs did not show any confirmative effects on the cell growth of HDPSCs although the initial cell adhesion events were stimulated on the nano-CPCs than on the micro-CPCs. In fact, some previous works on the behavior of osteoblastic cells cultured on similar CPC groups showed the cells had slightly lower proliferation levels on the nano-CPCs.15,19,28 It was suggested that the osteoblastic cell growth might be reduced on the nano-CPC due to the tight adhesion binding of cells to the underlying matrix, which consequently led to limited cell spreading and mitosis.15 Slightly different from that observation, here the nano-CPCs appeared not to delay the cell proliferation behaviors of the HDPSCs with respect to the micro-CPCs. The difference in cell type (osteosarcoma-derived cells vs. human dental pulp stem cells) and thus the different cellular adaptation to underlying substrate conditions may be a possible reason, which however, needs more clarification in future studies.
Rather than on the cell proliferation, the effects of the nano-sized CPCs were more clearly demonstrated on the odontogenic differentiation. The results of the ALP activity and the expression of marker genes such as BSP, OPN, OCN, DSPP, and DMP-1 indicated that nano-CPCs substantially promoted odontogenic differentiation when compared to micro-CPCs. ALP is mainly expressed on the cellular membrane of the osteoblasts and odontoblasts and is used as an important marker for the early differentiation cascade.29 Furthermore, DSPP and DMP-1 have been used as the odontogenic specific markers of HDPSCs while the OCN, OPN, and BSP genes are also expressed in osteogenic process.30 Therefore, analyses of these markers can inform whether the HDPSCs are sufficiently committed to an odontoblastic lineage.
The RT-PCR band intensities, when quantified, gave significant improvement in those series of odontogenic genes by the nano-CPCs, and this was more noticeable at the prolonged culture period of 14 days. It is considered that the HDPSCs exposed to the underlying CPC matrices for such a long period are considered to experience microenvironments quite different from those when simply cultured on tissue culture plastic. The nanotopological physical factor of CPCs should drive the stem cells to better conform to an odontogenic lineage with the proper supply of biochemical cues (OM medium). In addition to this topological physical factor, the chemical factor of possible ionic release is also considered to get involved in the odontogenic stimulation of HDPSCs. In fact, both CPC groups, even without the OM medium, led to substantial level of odontogenic differentiation and this was more noticeable at the prolonged culture period (14 days), when the ionic releases from CPCs would possibly be more pronounced. Although here we could not examine the ionic releases from CPCs, it is deduced that the nano-CPCs can release the ions at higher levels than the micro-CPCs due to the higher surface area since the ionic dissolution of calcium phosphates is surface reaction-controlled and thus surface area-dependent.
The integrin signaling pathway plays an important role in the cell–matrix interactions that control cell adhesion, survival, proliferation, and differentiation.31 The α1β1 and α2β1 integrins are the major adhesive molecule-binding receptors in cells that form bone and dentin, where α1β1 having a higher affinity for the basement membrane type IV collagen while α2β1 for the fibrillar type I collagen.32 Herein, the integrin subunits α1, α2, and β1 were shown to be expressed substantially in HDPSCs at the very early time points on both nano- and micro-CPCs, and the expression levels were higher when cultured on nano-CPCs than on micro-CPCs, implicating the HDPSCs recognized the nanotopological substrate much better than the microtopological one through the matrix receptors.
The downstream signaling of integrins may involve the activation of intracellular protein kinases, such as the focal adhesion kinase (FAK), paxillin, and Akt.33 In particular, the phosphorylation of FAK, paxillin and Akt was also shown to steadily increase in line with the integrin expressions. From a quantification, the HDPSCs on nano-CPCs expressed higher levels of p-FAK and p-Akt whilst lower levels of p-paxillin. It is unclear yet that the reason for the different stimulations between the intracellular down-streaming signals; it is postulated that other adhesion complex molecules that are regulated by the FAK might be involved more heavily than the paxillin, and regarding this more in-depth profiling of different sets of adhesion molecules will be needed. In fact, multiple integrin signaling pathways may interact through a variety of mechanisms, from membrane-proximal clustering of the two integrin types to the activation of a common downstream signaling pathways,34 thus the current data for the integrin-dependent yet non-harmonized FAK/paxillin/Akt activation by nano-CPCs also suggest the complex mechanistic events might contribute to signal enhancements in the nano-CPC-treated HDPSCs.
The downstream signaling of the intracellular molecules, mainly FAK, may involve the activation of MAPK and NF-κB.25 Our study demonstrated that the phosphorylation of MAPK and the activation of NF-κB were higher when cultured on nano-CPC than on micro-CPCs. These results indicate that the nano-CPCs might act through the FAK, Akt, MAPK pathways to induce NF-κB activation in the HDPSCs. The results are also consistent with previous studies on the bioceramic scaffolds or mineralized biopolymer scaffolds that promoted matrix-dependent differentiation of human osteoblasts through the MAPK signaling pathway34 and the activation of FAK, Akt, MAPK, and NF-κB.26 One consideration in the activation of NF-κB is that the NF-κB is involved in various signaling processes, not only in the osteogenic differentiation of mesenchymal stem cells,35–37 but also in the osteoclastic stimulation via RANK–RANKL signaling.38 In other words, the final results of NF-κB signaling in cells depend on what upstream factors activate it. Therefore, the ultimate activation of NF-κB should be understood in this context of positive as well as possible adverse facet, and the osteoclastic responses to the nano-CPCs may be a future interesting area of research. Within the limited findings, we could at least correlate the integrin-mediated adhesion signaling with the odontogenesis of HDPSCs, which was substantially stimulated on the nanotopological-tailored CPCs.
5. Conclusions
This is the first study that investigates the molecular signaling pathways through which nano-sized CPCs exert a substantial odontogenic stimulation of the HDPSCs. Our results demonstrate that nano-sized CPCs are superior to micro-sized CPCs in terms of their odontogenic differentiation in the HDPSCs through FAK, Akt, MAPK, and NF-κB signaling pathways. The results imply that the importance of particle size (particularly nano-sized particles) of cements that ultimately find usefulness as stem cell matrices and defect fillers for the endodontic repair and regeneration.Acknowledgements
This work was supported by grants from the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (no. 2012R1A5A2051384 & no. 2009-0093829).References
- Y. Sumita, M. J. Honda, T. Ohara, S. Tsuchiya, H. Sagara, H. Kagami and M. Ueda, Biomaterials, 2006, 27, 3238–3248 CrossRef CAS PubMed.
- W. H. Moolenaar, J. Biol. Chem., 1995, 270, 12949–12952 CrossRef CAS PubMed.
- M. L. Couble, J. C. Farges, F. Bleicher, B. Perrat-Mabillon, M. Boudeulle and H. Magloire, Calcif. Tissue Int., 2000, 66, 129–138 CrossRef CAS.
- Y. Miyamoto, K. Ishikawa, M. Takechi, T. Toh, Y. Yoshida, M. Nagayama, M. Kon and K. Asaoka, J. Biomed. Mater. Res., 1997, 37, 457–464 CrossRef CAS.
- R. A. Perez, S.-H. Shin, C.-M. Han and H.-W. Kim, Tissue Eng. Regener. Med., 2015, 12(3), 143–153 CrossRef CAS.
- R. A. Perez, K. Riccardi, G. Altankov and M.-P. Ginebra, J. Tissue Eng., 2014, 5 DOI:10.1177/2041731414543965.
- A. M. Cherng, L. C. Chow and S. Takagi, J. Endod., 2001, 27, 613–615 CrossRef CAS.
- A. Sugawara, L. C. Chow, S. Takagi and H. Chohayeb, J. Endod., 1990, 16, 162–165 CrossRef CAS.
- H. M. Chaung, C. H. Hong, C. P. Chiang, S. K. Lin, Y. S. Kuo, W. H. Lan and C. C. Hsieh, J. Formosan Med. Assoc., 1996, 95, 545–550 CAS.
- S.-K. Lee, S.-K. Lee, S.-I. Lee, J.-H. Park, J.-H. Jang, H.-W. Kim and E.-C. Kim, J. Endod., 2010, 36, 1537–1542 CrossRef PubMed.
- W.-J. Bae, S.-W. Chang, S.-I. Lee, K.-Y. Kum, K.-S. Bae and E.-C. Kim, J. Endod., 2010, 36, 1658–1663 CrossRef PubMed.
- B. Enkel, C. Dupas, V. Armengol, J. Akpe Adou, J. Bosco, G. Daculsi, A. Jean, O. Laboux, R. Z. LeGeros and P. Weiss, Expert Rev. Med. Devices, 2008, 5, 475–494 CrossRef CAS PubMed.
- S. S. Darwish, S. H. Abd El Meguid, N. A. Wahba, A. A. Mohamed, W. Chrzanowski and E. A. Abou Neel, J. Tissue Eng., 2014, 5 DOI:10.1177/2041731414521707.
- M. Espanol, R. A. Perez, E. B. Montufar, C. Marichal, A. Sacco and M. P. Ginebra, Acta Biomater., 2009, 5, 2752–2762 CrossRef CAS PubMed.
- E. Engel, S. Del Valle, C. Aparicio, G. Altankov, L. Asin, J. A. Planell and M.-P. Ginebra, Tissue Eng., Part A, 2008, 14, 1341–1351 CrossRef CAS PubMed.
- R. A. Perez, S. Del Valle, G. Altankov and M.-P. Ginebra, J. Biomed. Mater. Res., Part B, 2011, 97, 156–166 CrossRef PubMed.
- M. Otsuka, Y. Matsuda, Y. Suwa, J. L. Fox and W. I. Higuchi, J. Biomed. Mater. Res., 1995, 29, 25–32 CrossRef CAS PubMed.
- M. P. Ginebra, F. C. M. Driessens and J. A. Planell, Biomaterials, 2004, 25, 3453–3462 CrossRef CAS PubMed.
- R. A. Perez, G. Altankov, E. Jorge-Herrero and M. P. Ginebra, J. Tissue Eng. Regener. Med., 2013, 7, 353–361 CrossRef CAS PubMed.
- M. Kitagawa, H. Ueda, S. Iizuka, K. Sakamoto, H. Oka, Y. Kudo, I. Ogawa, M. Miyauchi, H. Tahara and T. Takata, Arch. Oral Biol., 2007, 52, 727–731 CrossRef CAS PubMed.
- J.-J. Kim, S.-J. Kim, Y.-S. Kim, S.-Y. Kim, S.-H. Park and E.-C. Kim, J. Endod., 2012, 38, 899–906 CrossRef PubMed.
- S.-Y. Lee, S.-Y. Kim, S.-H. Park, J.-J. Kim, J.-H. Jang and E.-C. Kim, J. Dent. Res., 2012, 91, 407–412 CrossRef CAS PubMed.
- E.-C. Kim, H.-J. Lee and Y. Kim, J. Endod., 2012, 38, 769–773 CrossRef PubMed.
- S.-J. Kim, K.-S. Min, H.-W. Ryu, H.-J. Lee and E.-C. Kim, J. Endod., 2010, 36, 1326–1331 CrossRef PubMed.
- S. Gronthos, K. Stewart, S. E. Graves, S. Hay and P. J. Simmons, J. Bone Miner. Res., 1997, 12, 1189–1197 CrossRef CAS PubMed.
- J.-J. Kim, W.-J. Bae, J.-M. Kim, J.-J. Kim, E.-J. Lee, H.-W. Kim and E.-C. Kim, J. Biomater. Appl., 2014, 28, 1069–1078 CrossRef CAS PubMed.
- W.-J. Bae, K.-S. Min, J.-J. Kim, J.-J. Kim, H.-W. Kim and E.-C. Kim, Dent. Mater., 2012, 28, 1271–1279 CrossRef CAS PubMed.
- R. A. Perez, T.-H. Kim, M. Kim, J.-H. Jang, M.-P. Ginebra and H.-W. Kim, J. Biomed. Mater. Res., Part A, 2013, 101, 923–931 CrossRef PubMed.
- H. Harris, Clin. Chim. Acta, 1990, 186, 133–150 CrossRef CAS.
- C. T. Hanks, D. Fang, Z. Sun, C. A. Edwards and W. T. Butler, Eur. J. Oral Sci., 1998, 106, 260–266 CrossRef CAS PubMed.
- P. J. Marie, Nat. Rev. Endocrinol., 2013, 9, 288–295 CrossRef CAS PubMed.
- J. Käpylä, J. Ivaska, R. Riikonen, P. Nykvist, O. Pentikäinen, M. Johnson and J. Heino, J. Biol. Chem., 2000, 275, 3348–3354 CrossRef PubMed.
- H. Xia, R. S. Nho, J. Kahm, J. Kleidon and C. A. Henke, J. Biol. Chem., 2004, 279, 33024–33034 CrossRef CAS PubMed.
- M. A. Schwartz and M. H. Ginsberg, Nat. Cell Biol., 2002, 4, E65–E68 CrossRef CAS PubMed.
- T. Wada, T. Nakashima, N. Hiroshi and J. M. Penninger, Trends Mol. Med., 2006, 12, 17–25 CrossRef CAS PubMed.
- H. H. Cho, et al., J. Cell. Physiol., 2010, 223, 168–177 CAS.
- K. Hess, A. Ushmorov, J. Fiedler, R. E. Brenner and T. Wirth, Bone, 2009, 45, 367–376 CrossRef CAS PubMed.
- X. Feng, et al., Cell Biol. Int., 2013, 37, 1267–1275 CrossRef CAS PubMed.
Footnote |
| † SY Lee and HM Yun contributed equally to this work as first authors. |
| This journal is © The Royal Society of Chemistry 2015 |





