Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dental lessons from past to present: ultrastructure and composition of teeth from plesiosaurs, dinosaurs, extinct and recent sharks†
A.
Lübke
a,
J.
Enax
a,
K.
Loza
a,
O.
Prymak
a,
P.
Gaengler
b,
H.-O.
Fabritius
c,
D.
Raabe
c and
M.
Epple
*a
aInstitute of Inorganic Chemistry and Center for Nanointegration Duisburg-Essen (CeNIDE), University of Duisburg-Essen, Universitaetsstr. 5-7, 45117 Essen, Germany. E-mail: matthias.epple@uni-due.de
bORMED, Institute for Oral Medicine at the University of Witten/Herdecke, Alfred-Herrhausen-Straße 45, 58448 Witten, Germany
cMicrostructure Physics and Alloy Design, Max-Planck-Institut für Eisenforschung, Max-Planck-Str. 1, 40237 Düsseldorf, Germany
First published on 13th July 2015
Abstract
Teeth represent the hardest tissue in vertebrates and appear very early in their evolution as an ancestral character of the Eugnathostomata (true jawed vertebrates). In recent vertebrates, two strategies to form and mineralize the outermost functional layer have persisted. In cartilaginous fish, the enameloid is of ectomesenchymal origin with fluoroapatite as the mineral phase. All other groups form enamel of ectodermal origin using hydroxyapatite as the mineral phase. The high abundance of teeth in the fossil record is ideal to compare structure and composition of teeth from extinct groups with those of their recent successors to elucidate possible evolutionary changes. Here, we studied the chemical composition and the microstructure of the teeth of six extinct shark species, two species of extinct marine reptiles and two dinosaur species using high-resolution chemical and microscopic methods. Although many of the ultrastructural features of fossilized teeth are similar to recent ones (especially for sharks where the ultrastructure basically did not change over millions of years), we found surprising differences in chemical composition. The tooth mineral of all extinct sharks was fluoroapatite in both dentin and enameloid, in sharp contrast to recent sharks where fluoroapatite is only found in enameloid. Unlike extinct sharks, recent sharks use hydroxyapatite as mineral in dentin. Most notably and hitherto unknown, all dinosaur and extinct marine reptile teeth contained fluoroapatite as mineral in dentin and enamel. Our results indicate a drastic change in the tooth mineralization strategy especially for terrestrial vertebrates that must have set in after the cretaceous period. Possibly, this is related to hitherto unconsidered environmental changes that caused unfavourable conditions for the use of fluoroapatite as tooth mineral.
Introduction
Teeth represent the hardest tissue in most living vertebrates. Their main function is catching prey and mastication of food.1 Therefore, they have a unique and delicate ultrastructure, typically with highly mineralized enamel on the outside and softer bone-like dentin inside representing the endodontium. Bony fish, amphibians, reptiles and mammals (including humans), use calcium phosphate as tooth mineral.2–5 The tooth mineral in vertebrates is hydroxyapatite with some carbonate substitutions on phosphate positions, the so-called dahllite.6 An exception are cartilaginous fish including sharks which use fluoroapatite as tooth mineral.7–9 We have shown recently that shark teeth contain fluoroapatite only in the outer layer, i.e. the enameloid (the enamel-equivalent in sharks, more appropriately called durodentin), but not in dentin.10,11 This enameloid is derived from cells of the tooth papilla and is different from true enamel of epithelial origin in bony fish and upper vertebrates.12Here we report on a comprehensive study of the teeth of extinct sharks, sauropterygians, mosasaurs and dinosaurs where their ultrastructure and chemical composition were analyzed with high-end chemical and microscopic methods (elemental analysis, scanning electron microscopy, X-ray powder diffraction including Rietveld refinement, infrared spectroscopy).
Experimental section
Analytical methods
Fossil teeth of the shark species S. pristodontus (found in Khouribga, Morocco), O. obliquus (found in Khouribga, Morocco), P. orientalis (found in Khouribga, Morocco), C. angustidens (found in South Carolina, USA), C. megalodon (found in South Carolina, USA), I. hastalis (found in Copiapo, Chile) as well as teeth of the extinct marine reptiles P. mauretanicus (found in Qued-Zem, Morocco) and M. beaugei (found in Qued-Zem, Morocco) and the dinosaurs S. maroccanus (found in Taouz, Morocco) and C. saharicus (found in Taouz, Morocco) were purchased from commercial sources specialized on fossils. The taxonomic determination of all species and their approximate time of extinction were kindly verified by Dr Witzmann, Museum für Naturkunde (Berlin, Germany) and Prof. Dr Kriwet, Naturkundemuseum (Vienna, Austria). Teeth of the recent shark species C. carcharias were kindly provided by the natural history museum in Vienna. An extracted human deciduous tooth (11 years) was also studied. Scanning electron microscopy (SEM) was used to visualize the internal structure of enameloid, enamel, dentin and the dentin–enameloid junction. The samples were prepared by cutting suitable pieces from the tooth crown using a jeweler's saw. Subsequently, each tooth piece was axially split using a sharp blade and a small hammer. The obtained tooth splinters were mounted onto standard aluminium SEM holders exposing the internal surfaces. All samples were rotary-shadowed with a 4 nm thick layer of platinum in a Gatan Precision Etching Coating System (PECS 682). SEM micrographs were recorded in a Zeiss Crossbeam XB1560 FIB-SEM at an acceleration voltage of 5 kV using a 30 μm aperture and an in-lens secondary electron detector at a working distance of 3 mm. Where necessary, contrast and brightness of the SEM micrographs were adjusted using Photoshop CS4 (Adobe Inc.).For axial freeze fracture, the teeth were immersed into liquid nitrogen for 2 min and mechanically broken into two pieces. For SEM in backscattering electron mode (BSE) the teeth were axially cut with a razor blade. Secondary electron (SE) microscopy and energy-dispersive X-ray spectroscopy (EDX) were carried out with an ESEM Quanta 400 FEG instrument after sputtering with gold and palladium (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). To determine the chemical nature, composition and the differences between enameloid, enamel and dentin regarding the crystallinity and the crystal size, infrared spectroscopy (IR) and X-ray powder diffraction (XRD) were used. For this, the samples were ground to a fine powder. The teeth were transversally cut using a Proxxon FBS 230/E fine drilling and polishing tool equipped with a diamond-coated cutting disk. Fine powders of enameloid, enamel and dentin (a few mg per sample), respectively, was obtained from corresponding areas of the cut teeth with the same instrument using a diamond-coated drill. The powder was used for infrared spectroscopy, X-ray diffraction, and elemental analysis. Fourier-transform infrared spectroscopy (FTIR) was carried out with a Bruker Vertex 70 instrument in KBr pellets (range 400–4000 cm−1 and 2 cm−1 resolution). X-ray powder diffraction was carried out with a Bruker D8 Advance diffractometer (Cu Kα radiation, λ = 1.54 Å), using a silicon single crystal sample holder to minimize background scattering.
20). To determine the chemical nature, composition and the differences between enameloid, enamel and dentin regarding the crystallinity and the crystal size, infrared spectroscopy (IR) and X-ray powder diffraction (XRD) were used. For this, the samples were ground to a fine powder. The teeth were transversally cut using a Proxxon FBS 230/E fine drilling and polishing tool equipped with a diamond-coated cutting disk. Fine powders of enameloid, enamel and dentin (a few mg per sample), respectively, was obtained from corresponding areas of the cut teeth with the same instrument using a diamond-coated drill. The powder was used for infrared spectroscopy, X-ray diffraction, and elemental analysis. Fourier-transform infrared spectroscopy (FTIR) was carried out with a Bruker Vertex 70 instrument in KBr pellets (range 400–4000 cm−1 and 2 cm−1 resolution). X-ray powder diffraction was carried out with a Bruker D8 Advance diffractometer (Cu Kα radiation, λ = 1.54 Å), using a silicon single crystal sample holder to minimize background scattering.
Rietveld refinement for the calculation of the lattice parameters and the crystallite sizes was performed with the Bruker software TOPAS 4.2. For each Rietveld refinement, the instrumental correction was included as determined with a standard powder sample of LaB6 (from NIST, National Institute of Standards and Technology, standard reference material, SRM 660b). The size of the crystallites was calculated with the Scherrer equation after correction for instrumental peak broadening.13 Elemental analysis was carried out to determine the overall chemical composition of the samples and a human deciduous tooth as reference and to confirm the identity of the mineral phases. For the determination of calcium, magnesium and sodium with atomic absorption spectroscopy (AAS), fluoride with ion-selective potentiometry and phosphate with ultraviolet (UV) spectroscopy, about 100 mg powder of dentin and enameloid were dissolved in concentrated hydrochloric acid. Calcium, sodium and magnesium were determined with a Thermo Electron, M-Series atomic absorption spectrometer. Phosphate was analysed with a Varian Cary 300 UV-Vis spectrophotometer as phosphate–molybdenum blue complex. For fluoride analysis we used ion-selective potentiometry (ion-selective electrode, ISE; pH/ION 735, WTW; measurement performed by Analytische Laboratorien GmbH, Lindlar, Germany).
The a-axes of the enameloid and of the geological fluoroapatite single crystal were shorter than that of synthetic hydroxyapatite. We have estimated the fluoride content using the correlation given by LeGeros and Suga.7 As this method assumes that the amount of foreign ions (like Na, Mg, carbonate) is negligible, these fluoride contents are associated with a considerable error. The presence of carbonate in the apatite lattice leads to a shortening of the a-axis, which results in higher fluoride-contents in the lattice using the LeGeros/Suga method.
Thermogravimetry (TG) was used to determine the contents of water, organic matrix and carbonated apatite in the teeth. For TG analysis, the teeth were transversally cut. To obtain pure enamel or enameloid, we cut off the tip of the tooth for analysis. To obtain almost pure dentin, we used the lower part of the tooth where it met the root. Thermogravimetry was carried out with a Netzsch STA 449 F3 Jupiter instrument in dynamic oxygen atmosphere (heating rate 2 K min−1 from 25 to 1200 °C; open alumina crucibles).
Results and discussion
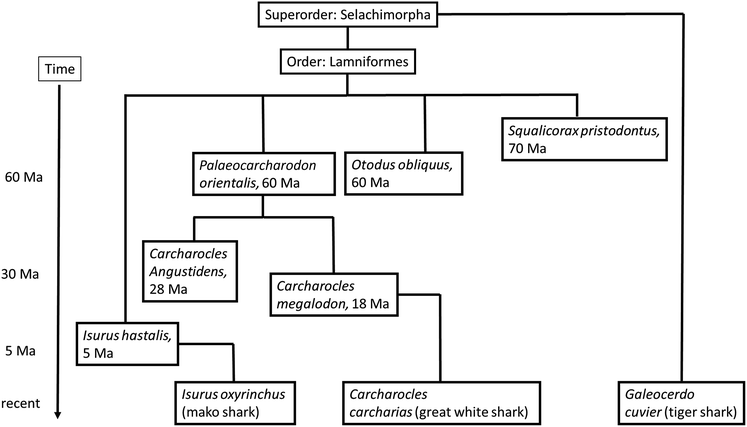
Sharks are cartilaginous fish, i.e. they have no bones. These chondrichthyans joined the evolution 460 million years ago. A remarkable difference to bony fish species is the fact that the mineral in their teeth is fluoroapatite, Ca5(PO4)3F, instead of hydroxyapatite, Ca5(PO4)3OH.14,15 The reason for the use of this different mineral is not known. It is not for the increased hardness of the mineral fluoroapatite compared to hydroxyapatite, as we have recently demonstrated that teeth of sharks (fluoroapatite) are not harder than human teeth (hydroxyapatite).10 This can be explained by the delicate ultrastructure of the outer layer of a tooth (enamel in vertebrates, enameloid in sharks), i.e. the arrangement of mineral crystals and organic matrix.11The teeth of extinct and recent sharks are easily available because they regularly shed off teeth due to their revolving rows of replacement teeth in the jaw. The phylogenetic relationship of the shark species that we have studied is shown in the ESI,† including Carcharocles megalodon, the extinct giant shark. Its estimated length was 15–20 m, based on the corresponding tooth size (note that in all fish, the tooth replacement leads to greater size following the growing size of the body), and its successor of today, Carcharodon carcharias, the great white shark.16
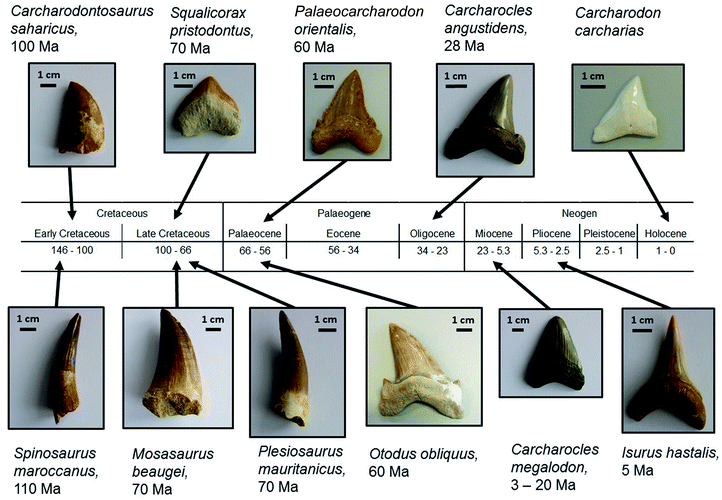
Plesiosaurs and mosasaurs are two groups of extinct marine reptiles that coexisted with the terrestrial saurischian dinosaurs. The tooth mineral of these animals is less well investigated. As they are extinct, a comparison to recent species is not possible as in the case of sharks. We have analysed the teeth of four species from two different phylogenetic groups. Plesiosaurus mauritanicus and Mosasaurus beaugei are both representatives of the Lepidosauromorpha and thus closely related to today's lizards and snakes. The dinosaurs Spinosaurus maroccanus and Carcharodontosaurus saharicus are Archosauromorpha and hence related to today's crocodiles and birds. These species were all carnivores that presumably all regularly changed their teeth. Fig. 1 shows images of all investigated teeth, including the age of the corresponding species whereas Fig. 2 shows the phylogenetic tree of all investigated shark species.
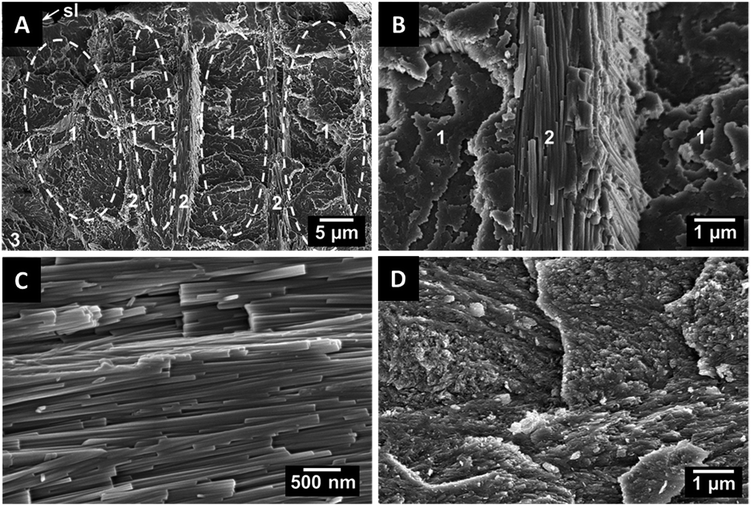
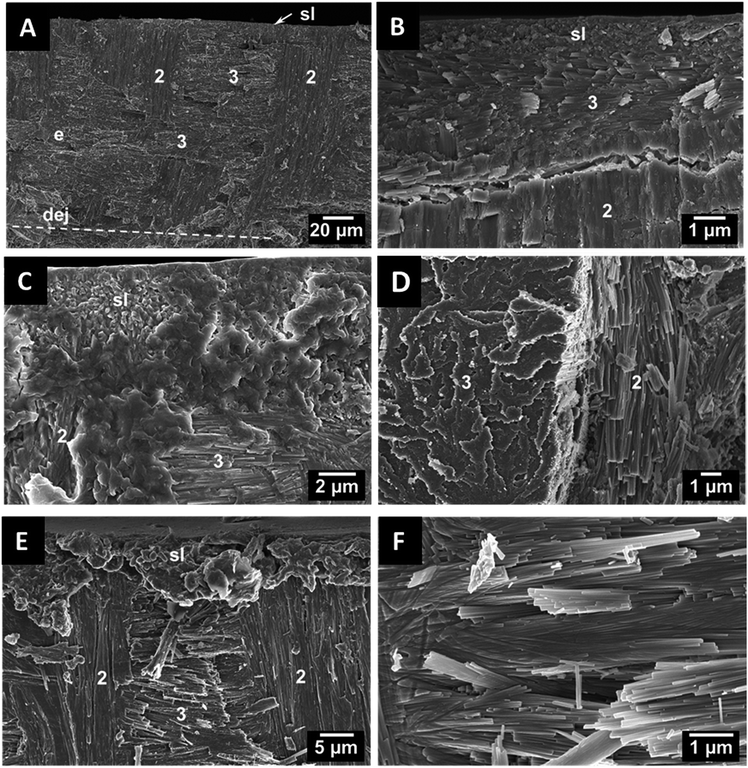
To explore the ultrastructure of dentin and enameloid by scanning electron microscopy, the teeth were axially fractured. In recent shark teeth, the larger enameloid crystallite bundles are interlaced with the fibres of the organic matrix which enhances the adhesion between the individual crystals and also between dentin and enameloid.11 In fossilized shark teeth, the organic matrix was not present due to degradation. As an example, a tooth of the fossil shark C. angustidens is shown in Fig. 3. Compared to the teeth of recent sharks,10,11 the tooth ultrastructure has been remarkably well preserved over the millions of years. The shiny layer, a thin surface layer with a random structure,15 is about 1 μm thick, and the enameloid has a thickness of about 100 μm. The enameloid of the shark species C. angustidens, Otodus obliquus, Palaeocarcharodon orientalis and Isurus hastalis (Fig. 4) consists of bundles of individual crystallites which are arranged in three different orientations with respect to the tooth geometry (radial, axial, longitudinal).
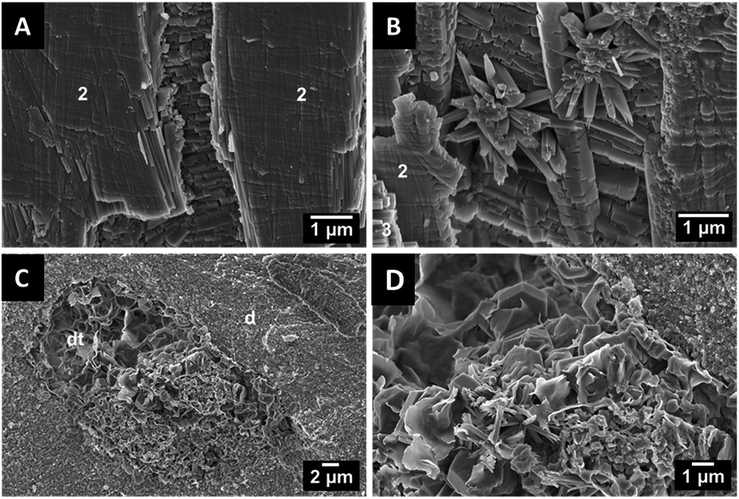
In teeth of other species like Squalicorax pristodontus, Carcharocles megalodon and the great white shark Carcharodon carcharias, only radial and axial bundles can be observed (Fig. 5). This anisotropic arrangement is likely to provide a higher mechanical strength and to improve the fracture toughness.17 The individual crystallites are rod-shaped with a length of a few μm and a diameter of about 100 nm. In some cases, we found artefacts in the dentin tubuli which result from the fossilization. As an example, the artefacts found in P. orientalis are shown in Fig. 6.
In general, the basic ultrastructural design of the fossilized shark teeth is almost identical to that of recent sharks and differs only in details such as the number and the distribution of circumferential bundles, if present. This suggests an optimized ultrastructure in shark teeth that withstood natural selection pressure without changing over time. Shark teeth represent a picivorous dentition, and they are optimally adapted to catch the prey or to crack mussels (in the case of rays). They have no masticatory function, therefore, there was no evolutionary “pressure” to develop enamel.
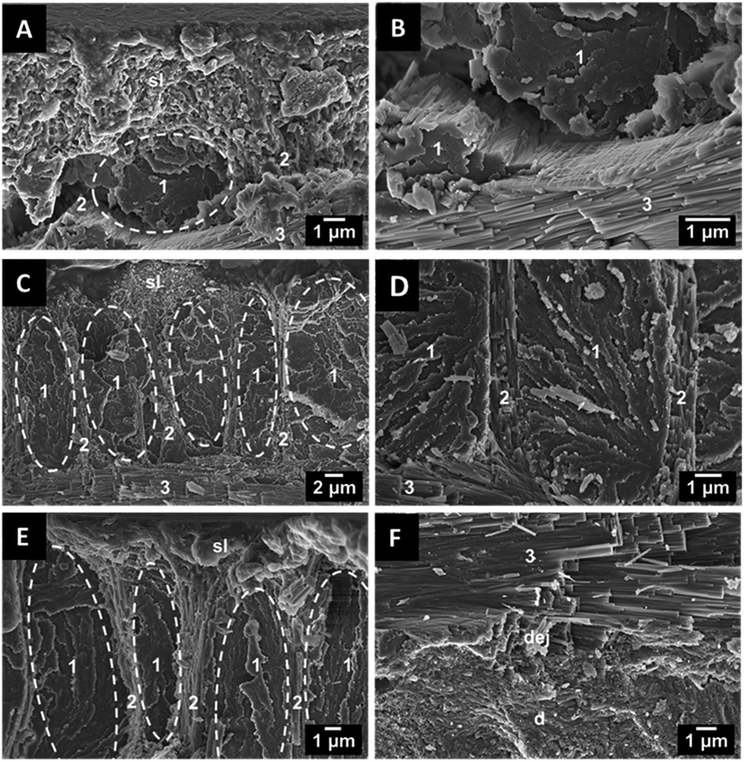
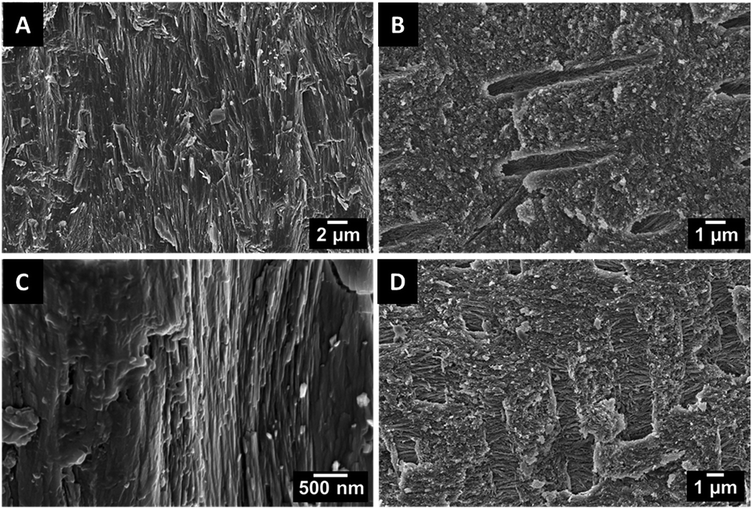
The teeth of the lepidosauromorph marine reptiles and the archosauromorph dinosaurs show the typical size and structure of most teeth of sauropsids. As there are no recent representatives, it cannot be decided to which extent the tooth structure has changed during fossilization. Because of the missing pulp, the fossilized shark teeth were much harder than dinosaur and plesiosaur teeth with extended pulp cavities. The latter tended to crumble during the mechanical cutting. Fig. 7 shows teeth of one representative species from each group. In the enamel, the crystallites closely resemble those forming the enameloid of extinct and recent sharks with a rod-shaped geometry and similar dimensions. However, they do not form distinct bundles and they all radially point outward, similar to the structure of human teeth. The dentin is characterized by mostly parallel tubules. Again, the tooth ultrastructure was remarkably well preserved.
Energy-dispersive X-ray spectroscopy (EDX) showed the elements oxygen, fluoride, phosphorus, and calcium with traces of sodium, magnesium and silicon in some cases. The presence of silicon can be ascribed to the diagenesis of the fossils. Sodium and magnesium are incorporated into the calcium phosphate of biological apatites.6 All fossilized teeth were fully mineralized, i.e. the organic matrix had decomposed over time, as shown by thermogravimetry.
Enamel, enameloid and dentin were chemically analysed in all cases with respect to the elements H, C, N, F, Na, Mg, P, S, and Ca. All fossilized teeth contained high amounts of calcium and phosphate and only traces of hydrogen, carbon, sodium, magnesium, and sulphur. Fluoride was present in variable amounts as we will discuss below. The results are given in Table 1, and thermogravimetric data and further elemental analysis data are given in Tables S2 and S3.†
| Ca2+ | PO43− | Ca/P molar ratio | Na+ | Mg2+ | F− (EA) | F− (XRD) | ||
|---|---|---|---|---|---|---|---|---|
| Fossil sharks | ||||||||
| S. pristodontus | Enameloid | 40.05 | 51.2 | 1.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.99 | 0.18 | 3.38 | 3.71 |
| Dentin | 40.55 | 45.35 | 2.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.91 | 0.12 | 3.56 | 5.28 | |
| O. obliquus | Enameloid | 37.6 | 51.1 | 1.73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.68 | 0.13 | 3.26 | 3.78 |
| Dentin | 38.1 | 45.3 | 1.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.76 | 0.13 | 3.27 | 3.94 | |
| P. orientalis | Enameloid | 40.15 | 50.4 | 1.88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.89 | 0.13 | 3.48 | 4.30 |
| Dentin | 39.75 | 44.65 | 2.11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.28 | 0.13 | 3.31 | 5.02 | |
| C. angustidens | Enameloid | 40.1 | 52.45 | 1.81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.89 | 0.15 | 3.27 | 2.91 |
| Dentin | 39.65 | 45.05 | 2.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.99 | 0.24 | 2.77 | 3.66 | |
| C. megalodon | Enameloid | 41.35 | 52.1 | 1.88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.72 | 0.17 | 3.24 | 4.23 |
| Dentin | 40.35 | 45.1 | 2.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.71 | 0.23 | 2.71 | 5.28 | |
| I. hastalis | Enameloid | 41.05 | 52.7 | 1.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.05 | >0.1 | 3.12 | 2.75 |
| Dentin | 39.7 | 43.35 | 2.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.86 | 0.14 | 2.65 | 3.78 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Recent sharks | ||||||||
| C. carcharias | Enameloid | 39.0 | 50.5 | 1.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.11 | 0.22 | 2.89 | 2.44 |
| Dentin | 23.15 | 40.4 | 1.36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.69 | 1.86 | 0.23 | n.d. | |
| I. oxyrinchus 10 | Enameloid | 37.81 | 54.35 | 1.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.03 | 0.13 | 3.08 | 3.44 |
| Dentin | 30.9 | 48.2 | 1.52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.14 | 0.44 | 0.61 | 2.21 | |
| G. cuvier 10 | Enameloid | 31.19 | 51.55 | 1.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.99 | 0.29 | 3.13 | 3.22 |
| Dentin | 24.26 | 41.8 | 1.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.35 | 0.82 | 1.46 | 2.21 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Dinosaurs | ||||||||
| S. maroccanus | Enamel | 39.4 | 45.8 | 2.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.09 | <0.1 | 1.29 | 1.99 |
| Dentin | 33.6 | 41.2 | 1.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.51 | <0.1 | 2.14 | 3.83 | |
| C. saharicus | Enamel | 38.5 | 45.2 | 2.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.91 | <0.1 | 1.72 | 3.61 |
| Dentin | 38.9 | 45.4 | 2.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.88 | <0.1 | 2.34 | 2.49 | |
| P. mauritanicus | Enamel | 39.5 | 46.1 | 2.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.61 | <0.1 | 1.56 | 2.77 |
| Dentin | 40.8 | 44.2 | 2.19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.69 | 0.16 | 3.19 | 5.56 | |
| M. beaugei | Enamel | 40 | 46.7 | 2.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.66 | 0.1 | 1.11 | 1.88 |
| Dentin | 41.5 | 45.3 | 2.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.8 | 0.11 | 2.94 | 4.50 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Humans | ||||||||
| Human teeth6 | Enamel | 36.0 | 54.3 | 1.57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.5 | 0.44 | 0.01 | |
| Dentin | 27.0 | 39.9 | 1.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.3 | 1.1 | 0.05 | ||
| Human deciduous tooth (molar) | <0.05 | |||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Minerals | ||||||||
| Geological fluoroapatite single crystal10 | 38.45 | 53.25 | 1.71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.18 | <3 × 10−6 | 3.64 | ||
| Stoichiometric fluoroapatite (computed) | 39.74 | 56.50 | 1.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 0 | 3.77 | ||
| Stoichiometric hydroxyapatite (computed) | 39.90 | 56.72 | 1.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 0 | 0 | ||
The crystallographic nature of the tooth mineral was assessed by X-ray powder diffraction. We found calcium phosphate as apatite in all cases, with a higher crystallinity in enamel and enameloid, and a lower crystallinity (or smaller crystallites) in dentin (Fig. 8). The crystallographic properties are listed in Table S1.† In the dentin of S. maroccanus and in the enamel of C. saharicus, we found traces of crystalline quartz due to diagenesis. Infrared spectroscopy and thermogravimetry confirmed the absence of organic material in all fossilized teeth.
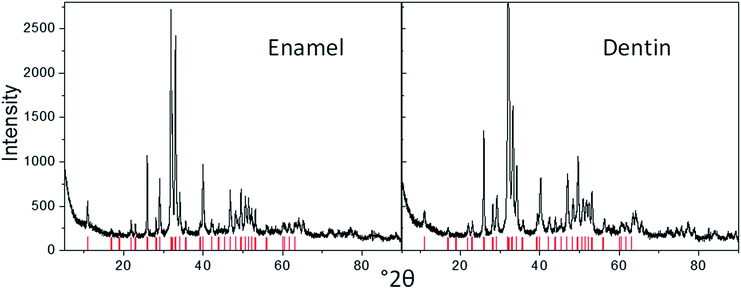 | ||
| Fig. 8 X-ray powder diffractograms of enamel and dentin in the tooth of the plesiosaur P. mauretanicus (the red bars show the positions of apatite according to the JCPDS Data Card. 09-0432). | ||
Thermogravimetry showed that there was almost no organic matrix left in the extinct species, neither in dentin nor in enamel and enameloid, confirming the elemental analysis data. The dentin of the recent shark C. carcharias contained 27.7% organic matrix, the enameloid contained 4.5% organic matrix.
The calcium content in all teeth as a function of fossilization is shown in Fig. 9. In all fossilized teeth, we detected a very high mineral content both in dentin and enamel. In teeth of recent species, dentin was less mineralized due to the presence of organic matrix.
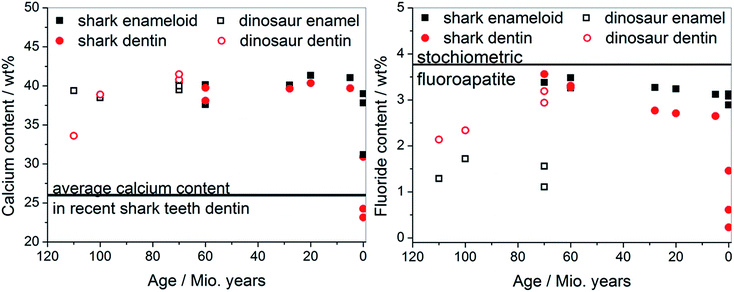 | ||
| Fig. 9 Correlation between the age of the extinct and recent species and the contents of calcium (left) and fluoride (right) (see Table 1 for the original data). | ||
The most surprising discovery was the fluoride content in the tooth mineral, in sharp contrast to recent human teeth (almost no fluoride) and recent shark teeth (fluoride only in the enameloid, but not in the dentin). The fluoride content was very high in all fossilized teeth, both for sharks, plesiosaurs, mosasaurs, and dinosaurs, both in dentin and in enamel. This suggests that the tooth mineral consists of fluoroapatite, Ca5(PO4)3F, instead of hydroxyapatite, Ca5(PO4)3OH. Both minerals have almost the same crystal structure and are nearly indistinguishable by X-ray diffraction, especially in the case of other substituting elements (like carbonate or sodium). We have refined apatite with a hexagonal lattice in all cases, as a fit to the monoclinic structure known for pure hydroxyapatite gave worse results. Therefore, elemental analysis represents the most accurate method to determine the mineral nature. The presence of any other crystalline fluoride-containing mineral (e.g. calcium fluoride, CaF2) was ruled out by X-ray diffraction.
The presence of fluoride in extinct reptile and dinosaur teeth is surprising, given the fact that recent sharks are the only major animal group which uses fluoroapatite as its main mineral phase, and even here it is used only in enameloid. Our results show that sharks have used fluoroapatite for their enameloid already in prehistoric times, and strongly suggest that this was the case also for dentin.
The plesiosaurs and mosasaurs represent two separate evolutionary branches within the same group Lepidosauromorpha, which have evolved into today's lizards and snakes. Both investigated species have also used fluoroapatite, both in enamel and dentin. The same applies to the representatives of their sister group Archosauromorpha, a lineage that includes today's crocodiles and birds. While the dinosaurs used fluoroapatite in both enamel and dentin, crocodiles use hydroxyapatite18 and birds have lost their teeth in favour of keratinous beaks.
Compared to calcium and phosphorus, fluoride occurs in rather low concentrations both in sea water (a few ppm)19 as well as in terrestrial environments. The fact that teeth from such very distantly related animal groups used the same fluoroapatite mineral composition for both enamel(oid) and dentin strongly suggests that during their lifetime in prehistoric times, the conditions, i.e. the abundance of fluoride, were more favourable than in recent times. While sharks have maintained fluoroapatite at least in the enameloid,8,10,11 the living relatives of the marine reptiles and dinosaurs have developed the use of hydroxyapatite as mineral for the entire tooth.1 This indicates that the change in conditions must have been more dramatic on land than in the sea. However, the reasons for this change in the abundance of fluoride are not known.
Another, albeit less probable reason for the presence of fluoroapatite in the dentin of all investigated teeth could be a hitherto undescribed chemical pathway where hydroxyapatite recrystallized to fluoroapatite during the millions of years of diagenesis. Given the fact that the measured fluoride content was high and similar on all extinct species despite their different age, excavation sites and diagenetic history, this appears very unlikely. Therefore, we rule out artefacts, e.g. of an intake of fluoride during fossilization, as the teeth were preserved in completely different environments and as this would not explain the pathway of fluoride inside all these fossilized teeth samples without changing the crystal morphology. In this respect, compact teeth are certainly different than porous dinosaur bones where a fluoride intake was reported by Elorza et al.20 Kohn et al. reported some chemical changes in fossil teeth, including some fluoride uptake.21 However, this was much less pronounced in enamel than in dentin. Therefore, unlike Bauluz et al. who studied the diagenesis of a tooth of an iguanodontian dinosaur to aluminium phosphate phases and proposed fluoroapatite as diagenetic phase,22 we are convinced that fluoroapatite was present in the teeth of the extinct species when they were still alive. These considerations are supported by EDX line scans across the teeth (see ESI†).
As mentioned above, the reason for the use of fluoroapatite by sharks in their teeth cannot be explained by an increased hardness.10 A better preservation against bacterial attack (caries) can also be excluded as sharks and marine reptiles regularly change their teeth. For land-living organisms such as the dinosaurs S. maroccanus and C. saharicus, it is particularly surprising that they have used fluoroapatite. This mineral dissolves in contact with acids (such as acidic fruits) below a pH of about 5.23 Contrary to hydroxyapatite as in human and mammalian teeth, it releases hydrofluoric acid (HF) which is a highly toxic compound. This will be rapidly diluted and removed for sea-living organisms such as sharks, but will remain in the mouth for land-living dinosaurs.
Conclusions
The ultrastructure of the teeth of sharks and dinosaurs has been remarkably well preserved during fossilization. In particular, the teeth of today's sharks still have the same ultrastructure as the teeth of extinct sharks. Differences were found in the tooth mineral which turned out to be fluoroapatite both in extinct sharks (enameloid and dentin) and dinosaurs (enamel and dentin). In contrast, recent sharks use fluoroapatite only in enameloid but not in dentin. Our results imply that a greater group not only of sea-living but also of land-living vertebrates has used fluoroapatite as tooth mineral. At some point during the evolution, the environmental circumstances may have changed and prevented the incorporation of fluoride into the apatite lattice of teeth.Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG) for generous support within the priority program 1420 (to M. E. and D. R.). We thank Mrs Carola Fischer for help with the sample preparation. We thank Jakob Epple for the donation of a deciduous tooth. We thank Dr Florian Witzmann, Museum für Naturkunde (Berlin, Germany) and Prof. Dr Juergen Kriwet, Naturkundemuseum (Vienna, Austria) for the taxonomic determination of the species and their approximate time of extinction. Teeth of the recent shark species C. carcharias were kindly provided by the Museum of Natural History in Vienna, Austria.References
- M. F. Teaford, M. M. Smith and M. W. J. Ferguson, Development, function and evolution of teeth, Cambridge University Press, Cambridge, 2000 Search PubMed.
- H. A. Lowenstam and S. Weiner, On biomineralization, Oxford University Press, New York, 1989 Search PubMed.
- S. Mann, Biomineralization, Oxford University Press, Oxford, 2001 Search PubMed.
- Handbook of Biomineralization, ed. E. Baeuerlein, P. Behrens and M. Epple, Wiley-VCH, Weinheim, 2007 Search PubMed.
- H. Ehrlich, Biological Materials of Marine Origin. Vertebrates, Springer, 2015 Search PubMed.
- R. Z. LeGeros, Prog. Cryst. Growth Charact., 1981, 4, 1–45 CrossRef CAS.
- R. Z. LeGeros and S. Suga, Calcif. Tissue Int., 1980, 32, 169–174 CrossRef CAS PubMed.
- G. Daculsi and L. M. Kerebel, Arch. Oral Biol., 1980, 25, 145–151 CrossRef CAS PubMed.
- L. B. Whitenack, D. C. Simkins Jr and P. J. Motta, J. Morphol., 2011, 272, 169–179 CrossRef PubMed.
- J. Enax, O. Prymak, D. Raabe and M. Epple, J. Struct. Biol., 2012, 178, 290–299 CrossRef CAS PubMed.
- J. Enax, A. M. Janus, D. Raabe, M. Epple and H. O. Fabritius, Acta Biomater., 2014, 10, 3959–3968 CrossRef CAS PubMed.
- M. M. Smith, Z. Johanson, C. Underwood and T. G. H. Diekwisch, Hist. Biol., 2012, 25, 127–142 CrossRef.
- H. P. Klug and L. E. Alexander, X-ray diffraction procedures for polycrystalline and amorphous materials, Wiley-Interscience, New York, 1974 Search PubMed.
- S. Suga, Y. Taki and K. Wada, Jpn. J. Ichthyol., 1982, 30, 81–93 Search PubMed.
- I. Sasagawa, Microsc. Res. Tech., 2002, 59, 396–407 CrossRef CAS PubMed.
- C. Pimiento and C. F. Clements, PLoS One, 2014, 9, e111086 Search PubMed.
- A. Al-Sawalmih, C. H. Li, S. Siegel, H. Fabritius, S. B. Yi, D. Raabe, P. Fratzl and O. Paris, Adv. Funct. Mater., 2008, 18, 3307–3314 CrossRef CAS.
- J. Enax, H. O. Fabritius, A. Rack, O. Prymak, D. Raabe and M. Epple, J. Struct. Biol., 2013, 184, 155–163 CrossRef CAS PubMed.
- R. Sen Gupta, S. Naik and S. Y. S. Singbal, Mar. Chem., 1978, 6, 125–141 CrossRef CAS.
- J. Elorza, H. Astibia, X. Murelaga and X. Pereda-Suberbiola, Cretaceous Res., 1999, 20, 169–187 CrossRef.
- M. J. Kohn, M. J. Schoeninger and W. W. Barker, Geochim. Cosmochim. Acta, 1999, 63, 2737–2747 CrossRef CAS.
- B. Bauluz, J. M. Gasca, M. Moreno-Azanza and J. I. Canudo, Lethaia, 2014, 47, 556–566 CrossRef.
- F. Lippert, D. M. Parker and K. D. Jandt, J. Colloid Interface Sci., 2004, 280, 442–448 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11560d |
| This journal is © The Royal Society of Chemistry 2015 |