3D hierarchical Co3O4 microspheres with enhanced lithium-ion battery performance†
Abstract
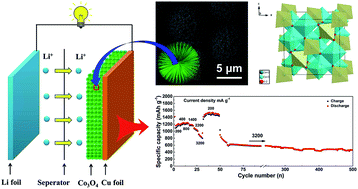
Although transition metal oxide electrodes have large lithium storage capacity, they often suffer from low rate capability and poor cycling stability. To develop an electrode with a long cycle life and good rate capability, 3D hierarchical tricobalt tetraoxide (Co3O4) spheres are fabricated under various hydrothermal conditions and evaluated as an anode in lithium-ion batteries. 3D hierarchical urchin-like Co3O4 electrodes exhibit a high reversible discharge capacity, excellent rate capability and good cycling performance, owing to their hierarchical architecture composed of micro-/nanostructures. Electrochemical testing shows that stable reversible capabilities of 1228 and 820 mA h g−1 can still be maintained after 170 cycles at 200 and 500 mA g−1, respectively. After rate capacity performance measurements, even the current density is increased to 3200 mA g−1 and a capacity of 587 mA h g−1 is retained after 500 cycles. The unique 3D hierarchical urchin-like Co3O4 electrode facilitates lithium ion diffusion and electron transportation and mitigates the internal mechanical stress induced by the volume variations of the electrode upon cycling, which lead to outstanding electrochemical performance.

 Please wait while we load your content...
Please wait while we load your content...