Synergistic effect of donors on tetracyanobutadine (TCBD) substituted ferrocenyl pyrenes†
Abstract
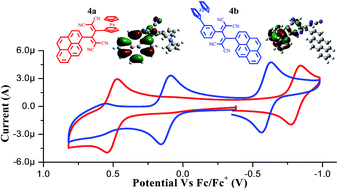
A set of ferrocenyl substituted pyrenes 2 and 3a–3c were designed and synthesized by the Pd-catalyzed Suzuki and Sonogashira cross-coupling reactions. The [2+2] cycloaddition–retroelectrocyclization reaction of ferrocenyl substituted pyrenes 3a and 3b with tetracyanoethylene (TCNE), resulted in tetracyanobutadine (TCBD) derivatives 4a, and 4b respectively. The synergistic effects of two donors (ferrocene and pyrene) and a TCBD acceptor on their photonic properties, energy levels and donor–acceptor interactions are evaluated. The photophysical and electrochemical properties of 2 and 3a–3c were compared with 4a and 4b. The ferrocenyl substituted pyrene 4b exhibits a strong charge transfer band from pyrene to TCBD and a weak charge transfer band from ferrocene to TCBD, whereas 4a exhibits two strong charge transfer bands, one from ferrocene to TCBD and another from pyrene to TCBD. The characteristic emission pattern of ferrocenyl substituted pyrenes 2, 3a–c, 4a and 4b indicates the emission from the pyrene moiety. The electrochemical properties reveal strong electronic communication between ferrocene and TCBD in 4a, and weak electronic communication in 4b. The experimental observations and conclusions were supported by computational calculations. The single crystal X-ray structure of ferrocenyl substituted pyrene 3b is reported.

 Please wait while we load your content...
Please wait while we load your content...