Cobalt hydroxide [Co(OH)2] loaded carbon fiber flexible electrode for high performance supercapacitor†
Ajay D. Jagadalea,
Guoqing Guan*ab,
Xiao Duac,
Xiaogang Haoc,
Xiumin Lib and
Abuliti Abudulaab
aNorth Japan Research Institute for Sustainable Energy (NJRISE), Hirosaki University, 2-1-3, Matsubara, Aomori 030-0813, Japan. E-mail: guan@hirosaki-u.ac.jp; Fax: +81 17 735 5411; Tel: +81 17 762 7756
bGraduate School of Science and Technology, Hirosaki University, 1-Bunkyocho, Hirosaki 036-8560, Japan
cDepartment of Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, P. R. China
First published on 24th June 2015
Abstract
Cobalt hydroxide nanoflakes are uniformly loaded on flexible carbon fiber (CF) paper, and provide good electrical connectivity to the current collector for use as supercapacitors. The unique porous nanostructure offers low ion diffusion and charge transfer resistance in the electrode. The effect of loading mass on electrochemical properties is investigated. The electrode with a mass loading of 2.5 mg cm−2 shows the maximum specific capacitance of 386.5 F g−1 at a current density of 1 mA cm−2. Also the same electrode provides a good rate capability with energy and power densities of 133.5 W h kg−1 and 1769 W kg−1, respectively even at a higher current density of 10 mA cm−2. The electrode reveals a cyclic stability of 92% over 2000 cycles. This kind of flexible, lightweight electrode could be effectively utilized for flexible supercapacitor fabrication, especially for wearable electronics.
1. Introduction
Electrochemical capacitors or supercapacitors have been attracting attention due to their promising properties of rendering high power and moderate energy densities without compromising cyclic stability. The fabrication of flexible, light weight and high performance supercapacitors is a prime requirement due to their potential application in wearable electronics, mobile phones and computers. Flexible supercapacitors play an important role in combining the high power of conventional capacitors and the high specific energy of batteries.1 Therefore, fabrication of flexible electrodes for supercapacitors is the most focused area of research. Flexible electrodes can be prepared using different current collectors, those that have been reported are stainless steel fibers (SSFs),2 aligned multi-walled carbon nanotubes (MWCNTs),3 carbon nanofiber (CNF) paper,4 and so on. Recently, several efforts have been made to prepare flexible supercapacitor electrodes, however some expensive strategies are used which provide an obstacle to commercializing such materials.5–7 Among these materials, flexible carbon microfiber (CF) is a superior current collector owing to its important properties such as low cost, good electric conductivity and good mechanical integrity.8 Also as it is a stick network, helps to maintain sufficient electron path.9 Previously, various CF supported electrodes have been reported for supercapacitor, Bao et al.10 have reported flexible Zn2SnO4/MnO2 core/shell nanocable-carbon microfiber electrode for supercapacitor and showed specific capacitance of 642.4 F g−1. Yu et al.11 have prepared reduced graphene oxide sheet wrapped polyaniline nanowire arrays on nitrogen-doped CF cloth for supercapacitor. Recently, Huang et al.12 have prepared a complex electrode made up of nickel–cobalt hydroxide nanosheets coated on NiCo2O4 nanowires grown on CF and reported the value of areal capacitance as high as 1.64 F cm−2 at 2 mA cm−2.On the other hand, cobalt hydroxide is a frequently reviewed candidate for supercapacitors with superior values of specific capacitance.13,14 Previously, cobalt hydroxide is reported with various nanostructures including nanoflakes,15 nanosheets,16 nanowires,17 nanoplatelets18 and nanorods.19 Among these, cobalt hydroxide with nanoflakes form has unique importance owing to its good electric conductivity, high surface area and good cyclic stability. However, these electrodes are inflexible and could be only utilized for rigid supercapacitor devices. There are very few reports on flexible electrodes formed with hydroxide coating. Warsi et al.20 have made conformal coating of cobalt–nickel layered double hydroxide nanoflakes on CFs for supercapacitor application. Besides, the crystal structure of cobalt hydroxide has been studied widely for supercapacitor application. Basically, it has been reported with two polymorphic phases as α and β-cobalt hydroxides. The α-Co(OH)2 phase is of hydrotalcite type having water molecules as well as anions like Cl−, NO3− and SO42− intercalated in the structure, while the β phase is an ordered staking of neutral Co(OH)2 layers.21 An intercalation of ions in the α-Co(OH)2 could improve the capacitive performance, because their sizes decide the interplanar spacing of basal plane. The enlarged spacing may lead to an enhancement in the transfer rate of proton through interaction between species,22 which could result in the excellent supercapacitive performance.
In the present work, the synergetic effect of pseudocapacitive properties of α-Co(OH)2 nanoflakes and flexible, good electrically conducting CFs is expected to achieve a flexible high performance supercapacitive electrode. As a part of this work, α-Co(OH)2 nanoflakes are uniformly loaded on the CF paper with different mass loadings using potentiodynamic electrodeposition method. The loaded samples are characterized using XRD, SEM and electrochemical analyses. An electrochemical tests of the electrodes have been made through cyclic voltammetry (CV), charging/discharging and electrochemical impedance spectroscopy techniques using three-electrode configuration. An effect of cobalt hydroxide mass loading on electrochemical properties has been systematically investigated.
2. Experimental
2.1 Materials and chemicals
CF paper (Torayca cloth, Toray Industries, Inc.) was used as a flexible substrate material. All chemical reagents were purchased from Wako Chemicals Inc., Japan and used as-received.2.2. Electrode fabrication
Initially, CF paper was cut into proper dimension. These pieces were cleaned with ethanol and distilled water, and then dried overnight in oven at 50 °C. Further, cobalt hydroxide was loaded onto these paper substrates in 0.1 M Co(NO3)2 solution using potentiodynamic electrodeposition method with potential window 0 to −1.3 V vs. Ag/AgCl at 50 mV s−1 scan rate. The loading masses were varied during potentiodynamic deposition method using potential cycles 20, 30, 40, 50 and 60 as 1.8, 1.9, 2.5, 2.9 and 3.1 mg cm−2, respectively. The detailed electrodeposition process is explained in our previous article published elsewhere.23 For the convenience, samples were labeled as CF20, CF30, CF40, CF50 and CF60, respectively. After mass loading, electrodes were rinsed with distilled water and then dried in oven at 50 °C for 1 h.2.3. Characterization
The morphology and structure of the electrodes were characterized with a scanning electron microscopy (SEM) (Hitachi SU6600, Japan). X-ray diffraction (XRD) patterns were recorded using a diffractometer (XRD 610, Shimadzu, Japan) equipped with a Cu Kα radiation source (λ = 1.5406 Å). The mass loadings were measured by weight difference method using electronic balance.2.4. Electrochemical measurement
All the electrochemical measurements were performed with a Solartron SI 1280B electrochemical measurement unit. A three-electrode set-up in 1 M LiOH was used for single electrode tests, with gauze platinum and Ag/AgCl as a counter and reference electrodes, respectively.3. Results and discussion
3.1 SEM
Fig. 1(a) displays photograph of CF paper coated with cobalt hydroxide nanoflakes which clearly shows dark bluish colored cobalt hydroxide layer coated on CF paper. Fig. 1(b) shows schematic diagram of actual mass loading process of cobalt hydroxide onto CF. Fig. 1(c) and (d) show SEM images of bare CF and cobalt hydroxide loaded CF. The size of bare CFs are few micrometers. It was uniformly covered by cobalt hydroxide nanoflakes after mass loading, the inset of Fig. 1(d) shows higher magnification of nanoflakes, estimates the flake thickness in the range of 15–20 nm.3.2 XRD
The crystal structure and type of polymorph of the cobalt hydroxide were examined by X-ray diffraction measurements. The formation of hexagonal type of cobalt hydroxide nanoflakes with α phase is confirmed, the illustrations for bare CF (a) and CF40 (b) are shown in Fig. 2. XRD patterns display noticeable peaks at around 10°, 19°, 33°, 59°, which are assigned to the (003), (006), (012), and (110) planes of the α-Co(OH)2, respectively. The diffraction data matches well with the data reported by Zhu et al. for the cobalt hydroxide fabricated by sonication assisted method.24 The pattern of bare CF sample shows two broad diffraction peaks around 2θ value of 24° and 43°, which may be attributed to the diffraction of (002) and (101) planes of graphite, respectively.25 The diffraction peak (012) has the typical broad “sawtooth” shape, indicates that the layer stacking in the α-Co(OH)2 might be loose or defective, which could lead to a turbostratic structure.26 It is a hydrotalcite type of structure with nitrate ions intercalated in the layers. Due to their comparatively smaller sizes they do not impend to OH− ions during the redox reactions at the interface, which certainly provides healthy environment for the redox reactions at the interface.223.3 Electrochemical measurement
Supercapacitive characteristics of α-Co(OH)2 loaded CF electrodes were performed by CV at various scan rates (Fig. 3). The capacitance mainly arises due to the redox reactions which might be seen from the oxidation and reduction peaks of CV curves. The detail surface faradaic reaction will be as,| Co(OH)2 + OH− ↔ CoOOH + H2O + e− | (1) |
To test the capacitive contribution of CF, we performed CV study for the bare CF electrode, but it was found negligible (figure is not shown). Fig. 3(a) shows CV curves for CF20, CF30, CF40, CF50 and CF60 electrodes at the scan rate of 5 mV s−1 in 1 M LiOH electrolyte within potential window −0.4 to +0.6 V vs. Ag/AgCl. Further, specific capacitance for each electrode was calculated from CV curves using formula,27
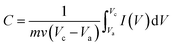 | (2) |
In order to evaluate the rate capability of electrodes, galvanostatic charge/discharge was performed at various current densities within a potential window 0 to +0.5 V vs. Ag/AgCl. Fig. 4(a) shows charge/discharge curves for CF20, CF30, CF40, CF50 and CF60 electrodes at the current density of 1 mA cm−2. The curves are non-linear, display pseudocapacitive behavior with increased discharging time for CF40 electrode. The specific capacitances are also calculated from the discharging branches using formula,
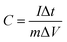 | (3) |
Furthermore, energy and power densities are calculated at the current density of 1 mA cm−2 for each electrode which is depicted in Fig. 4(b). The energy and power densities have been estimated from the following modified formulae,13
| ED = (ΔE × Id × Td/3600)/m | (4) |
| PD = ΔE × Id/m | (5) |
EIS was utilized to evaluate electrical parameters of α-Co(OH)2 loaded CF electrodes. Fig. 5(a) shows Nyquist plot for CF20, CF30 CF40, CF50 and CF60 electrodes in the frequency range from 20 kHz to 0.1 Hz. All electrodes show two semicircles in the high frequency region and an inclined line in the low frequency region. The intercept at the real axis in the high-frequency region is related to the internal resistance (Rs), which is a contribution of solution resistance, the intrinsic resistance of cobalt hydroxide and the contact resistance of electrode–electrolyte interface, while Rct results from the diffusion of electrons.37 The first semicircle is ascribed to the SEI (solid electrolyte interface) resistance and the second semicircle is attributed to the charge transfer resistance (Rct), while the inclined line at lower frequency region represents the diffusion of OH− ions within the pores of cobalt hydroxide. All electrodes depict the smaller diameters due to their good electrical conductivities and fast charge transfer reactions. Fig. 5(c) shows the equivalent circuit fitted for impedance data, the values of Rs and Rct for CF20, CF30, CF40, CF50 and CF60 electrodes are estimated as shown in Table 1. Table 1 depicts that Rs and Rct values increase with increase in loading mass. Here, CF40 exhibits Rs and Rct values as 2.51 and 1.66 Ω, respectively which are relatively lower than electrodes with higher mass loadings. The CF40 electrode with α-Co(OH)2 mass loading of 2.5 mg cm−2 provides lower resistive electronic diffusion and high surface area to achieve excellent supercapacitive performance. In the present study, the mass loading of an appropriate amount 2.5 mg cm−2 offers low-resistant pathways which might be produced due to short ion-diffusion channels. Furthermore, the cyclic stability of CF40 electrode was tested over 2000 number of charge/discharge cycles at the current density of 10 mA cm−2. Fig. 5(b) shows the variation of capacitance retention over the number of cycles, the stability is observed as 92%. The stability performance in the present case is superior to that of recently reported for cobalt hydroxide based materials29,32,38.
| Sample/parameter | Rs | Rct |
|---|---|---|
| CF20 | 2.34 | 1.36 |
| CF30 | 2.41 | 1.43 |
| CF40 | 2.51 | 1.66 |
| CF50 | 2.56 | 1.73 |
| CF60 | 3.52 | 3.50 |
To check applicability of electrodes, these electrodes further employed to fabricate flexible solid state supercapacitor. For this, the alkaline PVA/LiOH polymer electrolyte was prepared using a solution casting method. In a typical preparation, PVA (5 g) and LiOH·H2O (2 g) were mixed and dissolved in 50 mL of water with vigorous stirring for about 4 h at 85 °C. During supercapacitor fabrication, two electrodes with dimension 4 cm × 4 cm were prepared and soaked in the gel like polymer electrolyte for several hours. After soaking, these tow electrodes were stuck together under normal pressure (Fig. 6(a)). Finally the supercapacitor was dried at ambient temperature and used for testing. Flexibility of the supercapacitor was tested by recording CV curves at two different bending conditions. Fig. 6(b) shows CV curves of supercapacitor at bending conditions of 0° and 180° at scan rate of 20 mV s−1. It is clearly seen that the area under curve doesn't change significantly after bending which proves that supercapacitor is highly flexible and does not lose its structural integrity under bending conditions. Fig. 6(c) shows charging/discharging curves of supercapacitor at current densities of 0.5, 1 and 3 A g−1. Supercapacitor provides energy and power densities of 4.3 W h kg−1 and 424 W kg−1 at the current density of 3 A g−1. Fig. 6(d) reflects Nyquist plot for the supercapacitor within frequency range 105 Hz to 0.1 Hz. The intercept on the real axis is about 1.4 Ω which reveals good conductivity of the electrolyte and low internal resistance of the device.
4. Conclusions
In summary, α-Co(OH)2 loaded on CF paper with different loading amounts by potentiodynamic electrodeposition method. The effect of mass loading on electrochemical properties is investigated systematically. The CF40 electrode with a mass loading of 2.5 mg cm−2 shows the maximum specific capacitance of 386.5 F g−1 at the current density of 1 mA cm−2, also the same electrode provides the maximum energy density of 133.5 W h kg−1 with power delivery 1769 W kg−1 at the current density of 10 A g−1. The CF40 electrode has relatively low Rct value, can cater high power performance. The cyclic stability is observed as 92% for the mass loading of 2.5 mg cm−2. Solid state supercapacitor provides good electrochemical performance with excellent flexibility without losing structural integrity under bending conditions.Acknowledgements
This work is supported by the Japan Society for the Promotion of Science (JSPS).Notes and references
- Y.-Y. Horng, Y.-C. Lu, Y.-K. Hsu, C.-C. Chen, L.-C. Chen and K.-H. Chen, J. Power Sources, 2010, 195, 4418–4422 CrossRef CAS PubMed.
- J. S. Kim, S. S. Shin, H. S. Han, L. S. Oh, D. H. Kim, J. H. Kim, K. S. Hong and J. Y. Kim, ACS Appl. Mater. Interfaces, 2014, 6, 268–274 CAS.
- H. Lin, L. Li, J. Ren, Z. Cai, L. Qiu, Z. Yang and H. Peng, Sci. Rep., 2013, 3, 1353 Search PubMed.
- X. Yan, Z. Tai, J. Chen and Q. Xue, Nanoscale, 2011, 3, 212–216 RSC.
- X. Wang, B. Liu, Q. Wang, W. Song, X. Hou, D. Chen, Y. b. Cheng and G. Shen, Adv. Mater., 2013, 25, 1479–1486 CrossRef CAS PubMed.
- C. R. DeBlase, K. Hernandez-Burgos, K. E. Silberstein, G. G. Rodríguez-Calero, R. P. Bisbey, H. D. Abruña and W. R. Dichtel, ACS Nano, 2015, 9, 3178–3183 CrossRef CAS PubMed.
- C. Zhang, H. Yin, M. Han, Z. Dai, H. Pang, Y. Zheng, Y.-Q. Lan, J. Bao and J. Zhu, ACS Nano, 2014, 8, 3761–3770 CrossRef CAS PubMed.
- V. T. Le, H. Kim, A. Ghosh, J. Kim, J. Chang, Q. A. Vu, D. T. Pham, J. H. Lee, S. W. Kim and Y. H. Lee, ACS Nano, 2013, 7, 5940–5947 CrossRef CAS PubMed.
- L. Yang, S. Cheng, Y. Ding, X. Zhu, Z. L. Wang and M. Liu, Nano Lett., 2012, 12, 321–325 CrossRef CAS PubMed.
- L. Bao, J. Zang and X. Li, Nano Lett., 2011, 11, 1215–1220 CrossRef CAS PubMed.
- P. Yu, Y. Li, X. Zhao, L. Wu and Q. Zhang, Langmuir, 2014, 30, 5306–5313 CrossRef CAS PubMed.
- L. Huang, D. Chen, Y. Ding, S. Feng, Z. L. Wang and M. Liu, Nano Lett., 2013, 13, 3135–3139 CrossRef CAS PubMed.
- A. Jagadale, V. Kumbhar, D. Dhawale and C. Lokhande, Electrochim. Acta, 2013, 98, 32–38 CrossRef CAS PubMed.
- H. B. Li, M. H. Yu, X. H. Lu, P. Liu, Y. Liang, J. Xiao, Y. X. Tong and G. W. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 745–749 CAS.
- M. Li, S. Xu, C. Cherry, Y. Zhu, P. Yang, L. Wang and P. K. Chu, Electrochim. Acta, 2014, 149, 18–27 CrossRef CAS PubMed.
- V. Gupta, T. Kusahara, H. Toyama, S. Gupta and N. Miura, Electrochem. Commun., 2007, 9, 2315–2319 CrossRef CAS PubMed.
- H. Liu, K. H. Ho, Y. Hu, Q. Ke, L. Mao, Y. Zhang and J. Wang, Acta Mater., 2015, 84, 20–28 CrossRef CAS PubMed.
- J. Zhou, J. Li, K. Liu, L. Lan, H. Song and X. Chen, J. Mater. Chem. A, 2014, 2, 20706–20713 CAS.
- U. Patil, S. C. Lee, J. Sohn, S. Kulkarni, K. Gurav, J. Kim, J. H. Kim, S. Lee and S. C. Jun, Electrochim. Acta, 2014, 129, 334–342 CrossRef CAS PubMed.
- M. F. Warsi, I. Shakir, M. Shahid, M. Sarfraz, M. Nadeem and Z. A. Gilani, Electrochim. Acta, 2014, 135, 513–518 CrossRef CAS PubMed.
- J. R. Brownson and C. Lévy-Clément, Phys. Status Solidi B, 2008, 245, 1785–1791 CrossRef CAS PubMed.
- Z.-A. Hu, Y.-L. Xie, Y.-X. Wang, L.-J. Xie, G.-R. Fu, X.-Q. Jin, Z.-Y. Zhang, Y.-Y. Yang and H.-Y. Wu, J. Phys. Chem. C, 2009, 113, 12502–12508 CAS.
- A. Jagadale, V. Jamadade, S. Pusawale and C. Lokhande, Electrochim. Acta, 2012, 78, 92–97 CrossRef CAS PubMed.
- Y. Zhu, H. Li, Y. Koltypin and A. Gedanken, J. Mater. Chem., 2002, 12, 729–733 RSC.
- R. Elazari, G. Salitra, A. Garsuch, A. Panchenko and D. Aurbach, Adv. Mater., 2011, 23, 5641–5644 CrossRef CAS PubMed.
- Z. Liu, R. Ma, M. Osada, K. Takada and T. Sasaki, J. Am. Chem. Soc., 2005, 127, 13869–13874 CrossRef CAS PubMed.
- H. Li, M. Yu, F. Wang, P. Liu, Y. Liang, J. Xiao, C. Wang, Y. Tong and G. Yang, Nat. Commun., 2013, 4, 1894 CrossRef CAS PubMed.
- Z. Tai, J. Lang, X. Yan and Q. Xue, J. Electrochem. Soc., 2012, 159, A485–A491 CrossRef CAS PubMed.
- Z. Li, J. Wang, L. Niu, J. Sun, P. Gong, W. Hong, L. Ma and S. Yang, J. Power Sources, 2014, 245, 224–231 CrossRef CAS PubMed.
- J. Li, E.-h. Liu, W. Li, X.-y. Meng and S.-t. Tan, J. Alloys Compd., 2009, 478, 371–374 CrossRef CAS PubMed.
- L. Hu, W. Chen, X. Xie, N. Liu, Y. Yang, H. Wu, Y. Yao, M. Pasta, H. N. Alshareef and Y. Cui, ACS Nano, 2011, 5, 8904–8913 CrossRef CAS PubMed.
- Y. Tang, Y. Liu, S. Yu, S. Mu, S. Xiao, Y. Zhao and F. Gao, J. Power Sources, 2014, 256, 160–169 CrossRef CAS PubMed.
- X. Jiang, Y. Cao, P. Li, J. Wei, K. Wang, D. Wu and H. Zhu, Mater. Lett., 2014, 140, 43–47 CrossRef PubMed.
- T. Zhai, X. Lu, H. Wang, G. Wang, T. Mathis, T. Liu, C. Li, Y. Tong and Y. Li, Nano Lett., 2015, 15, 3189–3194 CrossRef CAS PubMed.
- Y. Tang, Y. Liu, S. Yu, W. Guo, S. Mu, H. Wang, Y. Zhao, L. Hou, Y. Fan and F. Gao, Electrochim. Acta, 2015, 161, 279–289 CrossRef CAS PubMed.
- Y. Bai, W. Wang, R. Wang, J. Sun and L. Gao, J. Mater. Chem. A, 2015, 3, 12530–12538 CAS.
- R. Rakhi, W. Chen, D. Cha and H. Alshareef, Nano Lett., 2012, 12, 2559–2567 CrossRef CAS PubMed.
- X. Cai, S. H. Lim, C. K. Poh, L. Lai, J. Lin and Z. Shen, J. Power Sources, 2014, 275, 298–304 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11366k |
| This journal is © The Royal Society of Chemistry 2015 |






