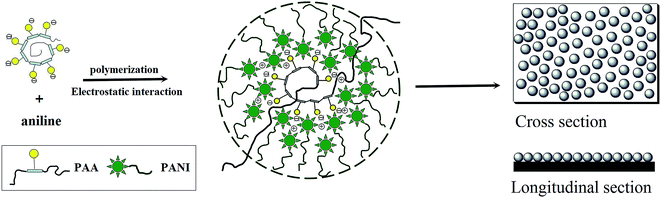
Micro/nanostructures of PANI obtained in the presence of water soluble polymers and their electrochemical sensing properties
Ting Wu,
Lu Yan Wang*,
Sen Du,
Wen Juan Guo and
Mei Shan Pei
School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China. E-mail: chm_wangly@ujn.edu.cn; Fax: +86-531-87161600; Tel: +86-531-89736800
First published on 6th August 2015
Abstract
Polyaniline (PANI) micro/nanostructures are synthesized by electrochemical polymerization, and characterized by field-emission scanning electron microscopy, Fourier transform infrared spectroscopy and UV-visible spectroscopy. It is revealed that the PANI microwires are produced with high yield with diameters of about 2 μm and lengths of several hundreds of micrometers, while nanostructures in the presence of PAA have diameters of about 500 nm. The micro/nanostructured PANI films are very stable and show high electrocatalytic reduction toward H2O2, which makes it an ideal substrate for H2O2 detection and offers great promise for biosensing applications.
1. Introduction
The combination of conductive polymers and nanotechnology has huge potential applications in catalysis, optics,1 transferring, sensors2–6 and microelectronics,7 due to the synergistic properties it will generate when the conductive polymers are made into nanostructures.8 Obtaining specific structures is the basis of the application of nanomaterials. Therefore, the preparation of conductive polymer nanomaterials with controllable morphology is very important. Many inorganic materials have well-defined thermodynamically or kinetically stable crystal structures that can be used to control their properties. However, conducting polymers lack a defined crystal structure that can be exploited in the same way. Instead, they generally rely on self-assembly processes determined by intermolecular interactions to form defined micro/nanostructures.1Among the numerous kinds of conducting polymers, polyaniline (PANI) is one of the most extensively studied due to its excellent electrical properties, good thermal and environmental stability, biocompatibility and easy fabrication process.9 In addition, PANI is cost-effective and flexible.10 The increasing importance is the ability of these materials to be synthesized in the form of micro/nanostructures.11–15 Compared with bulk PANI, polyaniline nanostructures have the properties of mechanical flexibility, high surface areas, chemical specificities, tunable conductivities and easy processing.16 Despite the current state-of-art reports, controllable preparation of highly ordered polyaniline micro/nanostructures is still a big challenge for fundamental research and practical applications.
In the field of preparation and assembly of conducting polymer nanomaterials, electrochemical methods have made many pioneering and practical research results.17,18 However, to control the ordered structure of conducting polymers at a molecular level, molecular ordering and orientation during the growth is an important and challenging topic. Until now, vacuum deposition, LB techniques, and electrospinning method are generally to fabricate micro/nanowires. But it is difficult for all these methods to realize aligned 1D structure with a controllable number of molecular layers. In this article, we show controllable growth of aligned microwires via a dip-coating method,20–24 which is a simple and easy control strategy to regulate the order of template molecules20,21 and realize aligned 1D structure. Water-soluble polymers have been employed in this work as templates,19–21 resulting to nanostructures being simple and easy to be controlled. The selection of the template is dependent on (a) the strong hydrogen bonding interaction between –OH and –NH2 which promote the self-assembly ability to form ordered and regular nano/microstructures, and (b) the ability of the series of compounds to exhibit excellent softness properties. Hydroxyethyl cellulose (HEC), polyacrylamide (PAM), chitosan (Cs) and polyacrylic acid (PAA) are chosen to be the template in this article. The combination of the functional groups of PANI and template molecules will lead to a significant modification in morphology, size and properties as well, of the final products. One of our aims here is to investigate the possibility of using water soluble polymers to obtain ordered PANI nanostructures in a controllable manner. Compared with other reported PANI nanowires, the obtained micro/nanowires in this work are all in highly ordered and have more advantages in the charge conduction than the other reported disordered PANI nanowires. In particular, aligned one-dimensional nanowire arrays of PANI have been regarded as ideal candidates for chemical sensor owing to their large specific area and optimized ion diffusion path. Moreover, the large specific surface area of PANI-nanostructures can provide a higher surface area for the interaction of target and facilitate the electron transfer. The obtained micro/nanostructured PANI films are very stable and show excellent electrocatalytic ability toward H2O2, especially the obtained of PANI–PAA nanowires. This characteristic can be used as an ideal substrate for H2O2 detection and offers great promise for biosensing.25
2. Experimental details
2.1 Materials
Aniline (≥99.5%) is purchased from Aladdin and distilled under reduced pressure prior to use. Lithium perchlorate (LiClO4), hydroxyethyl cellulose (HEC) and polyacrylic acid (PAA, molecular weight 3000) are also purchased from Aladdin Chemistry Co. Ltd Polyacrylamide (PAM, molecular weight 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and chitosan (Cs) are purchased from Sinopharm Chemical Reagent. De-ionized water is used to prepare all aqueous solutions.
000) and chitosan (Cs) are purchased from Sinopharm Chemical Reagent. De-ionized water is used to prepare all aqueous solutions.
2.2 Methods
All electrochemical measurements are performed on Zennium electrochemical workstation (Zahner, Germany). The micro-structures of PANI are characterized on a QUANTA PEG 250 scanning electron microscope (SEM). The FT-IR spectra of PANI products are recorded using Bruker VECTOR-22 spectrophotometer (using KBr pellet) in the range of 4000 to 200 cm−1 with a resolution of 2 cm−1. The UV-visible spectra of PANI and PANI nanostructure are measured using PerKinElmer Lambda 35. Fluorescence analysis are carried out with Edinburgh Instruments Ltd-FLS920 fluorescence spectrophotometer at room temperature. An OCA40 type of wetting angle measuring instrument made in German was used to measure sessile drop water contact angle, and the results are reported as the average of five measurements on different spots. The three-electrode electrochemical analysis was accomplished on a CHI 760E electrochemical working station (Chenhua Instruments Co., Shanghai, China), in which ITO glass (1 cm × 2 cm) is the working electrode, platinum wire is the counter electrode, and Ag/AgCl is the reference electrode. Before use, the working electrode is cleaned with acetone, then methanol followed by multiple rinses with deionized water.All electrochemical measurements are done in an unstirred electrochemical cell at room temperature.
2.3 Preparation of template solution
The HEC solution (0.01 wt%) is prepared by dissolving 0.01 g of HEC powder in 100 ml de-ionized water and stirred for 2 h at room temperature until a clear solution is obtained with a slight increase in viscosity. The preparation of PAM solution (0.01 wt%) is the same as that of the HEC solution. The Cs solution (0.01 wt%) is prepared by dissolving 0.01 g of Cs powder in 100 ml de-ionized water containing 1.2 ml acetic acid. Such solutions are used in the dip-coating approach to make the templates for preparing PANI microstructures.2.4 Fabrication of highly ordered PANI microstructures
In a typical dip-coating process,20,21 the ITO substrate (after hydrophilic treatment) is firstly immersed into the coating solution mentioned above for a few minutes and pulled out carefully and slowly. Then the template is easy prepared on the surface of the ITO glass. Subsequently, the template is dipped into the reaction solution containing aniline monomers, and the polymerization is initiated when electric current flows, with the prepared ITO glass covered with HEC template as the working electrode.A solution of template (10 ml) is added to a solution containing 0.1 M aniline in 10 ml H2O, LiClO4 is added as an electrolyte to polymerize the aniline monomers. The reaction time is ranging from 5 min to 20 min. Nitrogen is bubbled through the solution prior to deposition to remove oxygen and the polymerization is carried out under an inert nitrogen gas atmosphere. The aniline monomers are polymerized at a constant potential of 0.7 V. Aniline is distilled prior to being used. All the other reagents are used without further purification. Before the polymerization began, it is essential that aniline monomers has been absorbed with template molecules through hydrogen bonding for adequate time. In addition, in this work we introduce a simple but effective dip-coating approach for preparing highly ordered template on ITO surface, and then, high density and straight PANI nanowires were obtained by electrochemical deposition.
2.5 Fabrication of highly ordered PANI nanostructures
The process to produce PANI nanostructures is using organic acid doping technique (here the organic acid is PAA, molecular weight 3000). PAA (6 mM) is added directly to the aqueous electrolyte solution (20 ml) containing 0.1 M aniline and 0.14 M lithium perchlorate. When different potential are employed, the different nanostructures are obtained.3. Results and discussion
3.1 Morphology of PANI micro/nanostructure
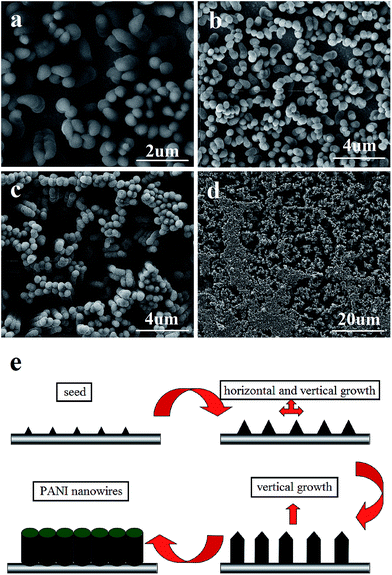
The morphology and size of the samples are analyzed by SEM. Fig. 1(a)–(c) shows the images of PANI microwires polymerized with HEC, PAM, and Cs molecules as templates, respectively, with diameters of 2 μm, 1 μm and 2 μm. It is worthy to notice that they are formed in high yields and arrayed in an orderly way. Water-soluble macromolecules, such as HEC,21 PAM, and Cs20 are chosen to be the template because of HEC, PAM and Cs molecules have chain shaped structures with many –OH groups and –NH2 groups, these groups can interact through hydrogen bonding interactions with –NH2 groups of aniline monomers. When the electrochemical synthesis is done without templates, the PANI films are grown randomly in orientation and size as shown in Fig. 2 (inset) and no wire is observed on the surface of the film. Such produced PANI–water soluble polymer (WSP) products show completely different morphologies from ordinary PANI (Fig. 2 (inset)). Therefore, the HEC, PAM and Cs molecules play an important role as the templates in the formation of PANI microwires. Our approach to make aligned microwires grown on ITO with templates is based on dip-coating process with slow pulling speed of the substrate. The mechanism of reaction is due to the stronger hydrogen bonds between template molecules and aniline monomers (Fig. 1(d)). Interestingly, the process is not only applicable for the production of polyaniline microfibers, but also other conducting polymer microfibers as well and the influencing factors of conducting polymers–water soluble polymers morphology in our previous work were investigate in detail.20Another kind of PANI nanostructure is produced by the polymerization of aniline monomers in the presence of poly(acrylic acid) (PAA). PAA26 as an organic acid and water soluble polymer, on the one hand can be doped aniline, on the other hand due to the electrostatic adsorption with aniline molecules can be used as a template. As shown in Fig. 3, the surface of PANI film is made of nanoparticles closely arranged with the size about 500 nm. In this work, anilinium cations ( ) being the prevailing species due to the presence of PAA(3K) in reaction medium. Aniline molecules and anilinium cations are two different in chemical properties. Anilinium cations have a higher oxidation potential than aniline molecules.26 Therefore when PAA is present, a high potential (1.5 V) are employed for the polymerization. The particles of such produced PANI film surface are uniform in size. Here PAA is a kind of negatively charged polyelectrolytes with strong hydrophilicity and easy to present a coiled state in aqueous solution. The present mechanism (Scheme 1) assumes that the aniline monomers are adsorbed on the chains of PAA by electrostatic interaction which seems to be the main driving force and subsequently the formed polyaniline nuclei, in the production of the nanoparticles on the PANI film surface. Furthermore, the hydrophobicity of the PANI nuclei is the key factor for the successful formation of closely packed nanospheres in aqueous media. When a higher potential is employed, a secondary growth of polyaniline is caused. Fig. 4(a) shows an SEM image of a PANI sample that is grown at 2.0 V for 1 min. It indicates that further growth of PANI has occurred along the vertical direction (perpendicular to the substrate surface). When a much longer deposition time of 5 min is utilized, the product morphology become wire-like, as indicated in Fig. 4(c). The lengths of the nanowires are about 4 micrometers. The image also shows that when the reaction time is prolonged, very little growth has occurred in the horizontal direction. The diameter of nanowires is 500 nm, which is consistent with the diameter size of nanoparticles presented in Fig. 3 and 4(a). This result illustrates that the subsequent polymerization of aniline monomers during a longer deposition time has predominantly occurred at the tips of the PANI particles shown in Fig. 4(a), extending the length of the nanowires further in the vertical direction (see Fig. 4(e)). This growth process is similar to the “seeding”27–31 growth process. Fig. 4(d) is a lower magnification image of the same sample as that shown in Fig. 4(c) and presents a larger surface areas.
) being the prevailing species due to the presence of PAA(3K) in reaction medium. Aniline molecules and anilinium cations are two different in chemical properties. Anilinium cations have a higher oxidation potential than aniline molecules.26 Therefore when PAA is present, a high potential (1.5 V) are employed for the polymerization. The particles of such produced PANI film surface are uniform in size. Here PAA is a kind of negatively charged polyelectrolytes with strong hydrophilicity and easy to present a coiled state in aqueous solution. The present mechanism (Scheme 1) assumes that the aniline monomers are adsorbed on the chains of PAA by electrostatic interaction which seems to be the main driving force and subsequently the formed polyaniline nuclei, in the production of the nanoparticles on the PANI film surface. Furthermore, the hydrophobicity of the PANI nuclei is the key factor for the successful formation of closely packed nanospheres in aqueous media. When a higher potential is employed, a secondary growth of polyaniline is caused. Fig. 4(a) shows an SEM image of a PANI sample that is grown at 2.0 V for 1 min. It indicates that further growth of PANI has occurred along the vertical direction (perpendicular to the substrate surface). When a much longer deposition time of 5 min is utilized, the product morphology become wire-like, as indicated in Fig. 4(c). The lengths of the nanowires are about 4 micrometers. The image also shows that when the reaction time is prolonged, very little growth has occurred in the horizontal direction. The diameter of nanowires is 500 nm, which is consistent with the diameter size of nanoparticles presented in Fig. 3 and 4(a). This result illustrates that the subsequent polymerization of aniline monomers during a longer deposition time has predominantly occurred at the tips of the PANI particles shown in Fig. 4(a), extending the length of the nanowires further in the vertical direction (see Fig. 4(e)). This growth process is similar to the “seeding”27–31 growth process. Fig. 4(d) is a lower magnification image of the same sample as that shown in Fig. 4(c) and presents a larger surface areas.
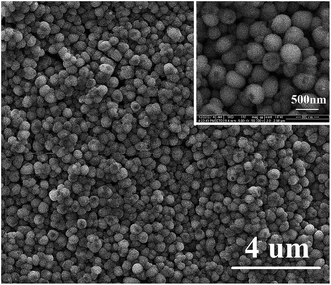 | ||
| Fig. 3 SEM images of PANI nanoparticles in the presence of PAA at 1.5 V potential for 5 min. Inset is the low magnification image. | ||
3.2 Characterization of PANI micro/nanostructures
The FT-IR spectra of PANI, PANI–HEC, PANI–PAM, PANI–Cs, PANI–PAA micro/nanostructures are shown in Fig. 2 and 5. The spectrum of PANI shows a prominent band at 3429 cm−1 due to N–H stretching vibration, bands at 1592 cm−1 and 1507 cm−1 due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of quinoid and benzenoid unit stretching modes, respectively. The bands at 1385 cm−1 and at 1312 cm−1 can be attributed to the C–N stretching vibration in (Q
C of quinoid and benzenoid unit stretching modes, respectively. The bands at 1385 cm−1 and at 1312 cm−1 can be attributed to the C–N stretching vibration in (Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q) unit and C–N stretching vibration of the secondary aromatic amine, respectively. The band at 1089 cm−1 and at 826 cm−1 can be assigned to C–H in-plane bending vibration and C–H out of plane bending vibration of para disubstituted benzene ring,32 respectively.
Q) unit and C–N stretching vibration of the secondary aromatic amine, respectively. The band at 1089 cm−1 and at 826 cm−1 can be assigned to C–H in-plane bending vibration and C–H out of plane bending vibration of para disubstituted benzene ring,32 respectively.
 | ||
| Fig. 5 FT-IR spectra of ordinary PANI, PANI–HEC, PANI–PAM, PANI–Cs, PANI–PAA nanoparticles (NPs) and PANI–PAA nanowires (NWs). | ||
The FT-IR spectra of PANI water-soluble polymer (WSP) nanostructures show all the significant characteristic bands of PANI in the position of the bands. The bands at 2931 cm−1 and 2856 cm−1 correspond to C–H stretching of aliphatic group.33 The absorption band at 1632 cm−1 can be attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (the part of PAM and PAA). The N–H bending in primary amine groups (–NH2) are observed clearly at 1592 cm−1. Other major bands observed in PANI–WSP are found at 1158 cm−1 (anti-symmetrical stretching vibration of the C–O–C bridge). Furthermore, the shifting of the band at 3429 cm−1 in PANI (Fig. 2), being assigned to the stretching vibration of N–H groups, to 3452 cm−1 is observed. The broadening and the shifting of the latter band can be assigned to the electrostatic forces (–NH3+ and –COO−) and the hydrogen bonding between the moieties of water-soluble polymers and PANI (–NH2 and –OH groups). There is no clear indication for covalent bond formation or grafting of WPS onto PANI as revealed by Ma et al.34 In accordance with the aforementioned IR results, it can be concluded the electrostatic forces and hydrogen bonding are the driving forces between the moieties of PANI and water-soluble polymers.
O stretching (the part of PAM and PAA). The N–H bending in primary amine groups (–NH2) are observed clearly at 1592 cm−1. Other major bands observed in PANI–WSP are found at 1158 cm−1 (anti-symmetrical stretching vibration of the C–O–C bridge). Furthermore, the shifting of the band at 3429 cm−1 in PANI (Fig. 2), being assigned to the stretching vibration of N–H groups, to 3452 cm−1 is observed. The broadening and the shifting of the latter band can be assigned to the electrostatic forces (–NH3+ and –COO−) and the hydrogen bonding between the moieties of water-soluble polymers and PANI (–NH2 and –OH groups). There is no clear indication for covalent bond formation or grafting of WPS onto PANI as revealed by Ma et al.34 In accordance with the aforementioned IR results, it can be concluded the electrostatic forces and hydrogen bonding are the driving forces between the moieties of PANI and water-soluble polymers.
UV-vis spectra are also used to determine the formation of PANI. Fig. 6 shows UV-vis spectra for PANI, PANI–WSP nanostructures in ethanol. The PANI absorption peaks being observed at about 282 and 550 nm, correspond to π–π transition of the benzenoid ring and π–π transition of quinoid rings,35 respectively. The absorption peaks of PANI–WSP nanostructures appear at about 306 and 560 nm which are similar to the peaks observed for PANI with a little red shift. The red shift of the maximum absorption peaks indicates the weak interaction of water soluble polymers with PANI. And also it confirms the conclusion that the interaction between water soluble polymers and PANI is due to electrostatic interaction and hydrogen bond between the moieties of water-soluble polymers and PANI, as previously concluded from the IR analysis. The fluorescence properties of obtained PANI micro/nanostructures are further investigated. As can be seen clearly from Fig. 7, the fluorescence intensity of PANI micro/nanostructures are higher than that of the ordinary PANI film. Especially among these curves, the fluorescence curve of PANI–PAA NWs presents the highest intensity at about 400 nm.
 | ||
Fig. 6 The UV-vis absorption spectra of PANI, PANI–HEC, PANI–PAM, PANI–Cs, PANI–PAA NPs and PANI–PAA NWs (solvent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol). ethanol). | ||
 | ||
Fig. 7 The fluorescence spectra of (a) ordinary PANI. (b) PANI–HEC, (c) PANI–PAM, (d) PANI–Cs, (e) PANI–PAA NPs and (f) PANI–PAA NWs, respectively (solvent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol). ethanol). | ||
Fig. 8 represents the contact angle measurement images of ordinary PANI film, PANI–HEC, PANI–PAM, PANI–Cs, PANI–PAA nanoparticles (NPs) and PANI–PAA nanowires (NWs). The contact angle of water at ITO glass surface is 48.8° (the picture is not shown). A increase in contact angle (96.8°) is noticed with a deposition of ordinary PANI, which is due to the hydrophobicity of PANI. Likewise, the contact angles for water of PANI–HEC, PANI–PAM and PANI–Cs are kept at 81.3°, 67.2° and 78.9°, respectively, which is lower that of ordinary PANI (96.8°). This should be attributed to the water soluble polymers combined in the PANI micro/nanostructures. Thus the existence of WSPs can successfully tune the surface hydrophilicity of PANI. However, on the surfaces of PANI–PAA NPs and PANI–PAA NWs, the contact angles become much higher (115.7° and 127.4°, respectively) than that on the ordinary PANI surface. This is because of the changes in surface roughness between ordinary PANI and PANI–PAA, as can be noticed in SEM images (Fig. 2 (inset) and 4). The surfaces of PANI–HEC, PANI–PAM and PANI–Cs are more hydrophilic in comparison to PANI, and the surface of PANI–PAA are more hydrophobic in comparison to PANI. Therefore, the application of polyaniline can be more extensive.
 | ||
| Fig. 8 Photographs of water drop on ordinary PANI (a), PANI-HEC (b), PANI-PAM (c), PANI-Cs (d), PANI-PAA NPs (e) and PANI-PAA NWs (f) deposited on the glass substrate, respectively. | ||
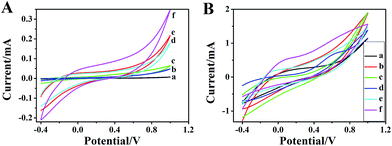
Cyclic voltammograms are obtained to investigate the electromotive of PANI and PANI nanostructures over a potential range from −0.2 to 0.9 V. Fig. 9 describes the typical cyclic voltammograms of PANI film with different micro/nanostructures in 1 M HCl solution containing 1 M NaCl at a scan rate of 100 mV s−1. It can be seen clearly from Fig. 9 that PANI micro/nanostructures films show very good electromotive in 1 M HCl solution containing 1 M NaCl. Comparing the six CV curves, it can be concluded that the peak currents of the PANI micro/nanostructures are much higher than that of the ordinary PANI, with cathodic peak at ca. −70 mV and the corresponding anodic peak at ca. 750 mV. This can be ascribed to the high surface area of PANI micro/nanostructures, which enlarges the PANI/electrolyte interface. Especially, among these nanostructures synthesized from different techniques, those produced by PAA-doped process (as shown in Fig. 4(d)) have the highest electrolyte capacitive charges, which is ascribed to the high surface area of nanostructural film.
 | ||
| Fig. 9 Cyclic voltammograms of (a) ordinary PANI, (b) PANI–HEC, (c) PANI–PAM, (d) PANI–Cs, (e) PANI–PAA NPs, (f) PANI–PAA NWs films in 1 M HCl solution containing 1 M NaCl. Scan rate: 100 mV s−1. | ||
3.3 Electrocatalysis determination of H2O2
Characterized by an interesting type of micro/nanostructures with large surface areas, PANI micro/nanostructures are favorable for the unlimited transport of molecules and electron conductivity. Consequently, it is interesting to explore the electrocatalytic activity of H2O2 electrooxidation over the PANI micro/nanostructures. Fig. 10 shows the cyclic voltammetric (CV) curves of ordinary PANI and PANI micro/nanostructures in 0.1 M PBS solution (pH 7.0) in the presence (Fig. 10(B)) and absence (Fig. 10(A)) of H2O2. When 0.5 mM H2O2 is added in PBS solution (pH 7.0), noticeable increase of redox currents could be observed, indicating a high catalytic activity of PANI micro/nanostructures toward H2O2. In the blank PBS solution (pH 7.0), there is no obvious current change(Fig. 10(A)). Among the micro/nanostructures of PANI, the PANI-PAA nanowires shows the highest catalytic activity towards H2O2.The electrocatalytic ability of PANI–PAA nanowires is investigated in detail. As shown in Fig. 11, with a further increase of H2O2 concentration, the oxidation current dramatically rises. For electrochemical sensing applications, the sensing performance of electro-catalysts is usually evaluated by measuring the current response at fixed potentials versus time after the addition of analytes. Fig. 12 shows the amperometric responses of PANI–PAA nanowires (working electrode is a polished glassy carbon electrode (GCE, 3 mm in diameter). The morphology of PANI on GCE are shown in Fig. 12(B) (inset), which is the same as Fig. 4) on successive addition of H2O2 into the stirring PBS solution at 0.7 V applied potentials. In order to clear observation, the current responses on lower concentration of H2O2 solution are enlarged as shown in the inset of Fig. 12(A). Under the 0.7 V working potential, the response currents of H2O2 at PANI–PAA nanowires electrode are linear with their concentrations range from 0.5 mM to 11 mM, indicating that PANI–PAA nanowires exhibit high current response to the addition of H2O2 and rapidly reach the maximum steady-state currents within 1 s. The fast response on PANI–PAA nanowires can be attributed to the high surface area and microporous structure of the nanoscaled film. The calibration curves (Fig. 12(B)) indicate that the PANI–PAA NWs have linear responses to H2O2 concentration in the range of 0.5–11 mM (linear equation: y = 0.146 + 2.5x, R = 0.998).
 | ||
| Fig. 11 CV curves of PANI–PAA nanowires film in PBS, and PBS + H2O2 mixed solution with H2O2 concentrations of 0.5 (b), 1 (c), 2 (d), 3 (e), and 5 (f) mM. Scan rate 100 mV s−1. | ||
4. Conclusions
In summary, the PANI microwires can be generated by electropolymerization of aniline through different water-soluble macromolecule templates (HEC, PAM and Cs) by a dip-coating process. The PANI nanoparticles and nanowires are also obtained under different conditions, respectively, in the presence of PAA. Those structures (especially the PANI–PAA nanowires) show evidently good electrochemical properties, due to their orderly arranged wire-like microstructures and large surface areas. As a result of the improved electrochemical performance, the PANI macro/nanostructures films show an excellent electrocatalytic activity for H2O2 detection without any activation pretreatment. During the biosensing detection, the PANI–PAA NWs present fast response and large oxidation current toward H2O2. Consequently, the PANI micro/nanostructures hold excellent catalytic performance, simple synthesis process, low cost and long-term stability.Acknowledgements
This work is supported by the National Natural Science Foundation of China (51003040), Shandong Provincial Natural Science Foundation, China (ZR2012BL11), and Shandong Provincial Science and Technology Development Plan Project, China (2013GGX10705).References
- R. H. Friend, R. W. Gymer, A. B. Holmes and J. H. Burroughes, Electroluminescence in conjugated polymers, Nature, 1999, 397, 121–128 CrossRef CAS.
- N. T. Kemp, J. W. Cochrane and R. Newbury, Characteristics of the nucleation and growth of template-free polyaniline nanowires and fibrils, Synth. Met., 2009, 159, 435–444 CrossRef CAS PubMed.
- J. Huang, S. Virji, B. H. Weiller and R. B. Kaner, Polyaniline nanofibers: facile synthesis and chemical sensors, J. Am. Chem. Soc., 2003, 125, 314–325 CrossRef CAS PubMed.
- H. Liu, J. Kameoka, D. A. Czaplewski and H. G. Craighead, Polymeric nanowire chemical sensor, Nano Lett., 2004, 4, 671–675 CrossRef CAS.
- N. T. Kemp, A. B. Kaiser, H. J. Trodahl and B. Chapman, Effect of ammonia on the temperature-dependent conductivity and thermopower of polypyrrole, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 1331–1338 CrossRef CAS PubMed.
- P. K. Kahol, N. T. Kemp and A. B. Kaiser, An electron paramagnetic resonance study of morphological disorder in polypyrrole through oxygen effects, Solid State Commun., 2005, 135, 775–779 CrossRef CAS PubMed.
- J. G. Killian, B. M. Coffey, F. Gao and T. O. Poehler, Polypyrrole composite electrodes in an all-polymer battery system, J. Electrochem. Soc., 1996, 143, 936–942 CrossRef CAS PubMed.
- H. D. Tran, J. M. D'Arcy, Y. Wang, P. J. Beltramo, V. A. Strongb and R. B. Kaner, The oxidation of aniline to produce “polyaniline”: a process yielding many different nanoscale structures, J. Mater. Chem., 2011, 21, 3534–3550 RSC.
- X. F. Yu, Y. X. Li and K.-Z. Kourosh, Synthesis and electrochemical properties of template-based polyaniline nanowires and template-free nanofibril arrays: Two potential nanostructures for gas sensors, Sens. Actuators, B, 2009, 136, 1–7 CrossRef CAS PubMed.
- L. Z. Fan and J. Maier, High-performance polypyrrole electrode materials for redox supercapacitors, Electrochem. Commun., 2006, 8, 937–940 CrossRef CAS PubMed.
- H. D. Tran, D. Li and R. B. Kaner, One-Dimensional Conducting Polymer Nanostructures: Bulk Synthesis and Applications, Adv. Mater., 2009, 21, 1487–1499 CrossRef CAS PubMed.
- (a) M. X. A. Wan, Template-Free Method Towards Conducting Polymer Nanostructures, Adv. Mater., 2008, 20, 2926–2932 CrossRef CAS PubMed; (b) M. X. Wan, Some Issues Related to Polyaniline Micro-/Nanostructures, Macromol. Rapid Commun., 2009, 30, 963–975 CrossRef CAS PubMed.
- (a) J. X. Huang and R. B. Kaner, The intrinsic nanofibrillar morphology of polyaniline, Chem. Commun., 2006, 367–376 RSC; (b) D. Li, J. X. Huang and R. B. Kaner, Polyaniline Nanofibers: A Unique Polymer Nanostructure for Versatile Applications, Acc. Chem. Res., 2009, 42, 135–145 CrossRef CAS PubMed.
- J. Jang, Conducting polymer nanomaterials and their applications, Adv. Polym. Sci., 2006, 199, 189–259 CrossRef CAS.
- D. H. Zhang and Y. Y. Wang, Synthesis and applications of one-dimensional nano-structured polyaniline: An overview, Mater. Sci. Eng., B, 2006, 134, 9–19 CrossRef CAS PubMed.
- B. Yang, H. Y. Yang, Y. Hu, T. M. Yao and S. S. Huang, A novel electrochemical DNA biosensor based on graphene and polyaniline nano wires, Electrochim. Acta, 2011, 56, 2676–2681 CrossRef PubMed.
- K. Wang, J. Y. Huang and Z. X. Wei, Conducting Polyaniline Nanowire Arrays for High Performance Supercapacitors, J. Phys. Chem. C, 2010, 114, 8062–8067 CAS.
- Q. Cheng, J. Tang, J. Ma, H. Zhang, N. Shinya and L. C. Qin, Graphene and nanostructured MnO2 composite electrodes for supercapacitors, J. Phys. Chem. C, 2011, 49, 2917–2925 CAS.
- F. Miculescu, E. Rusen, A. Mocanu, A. Diacon and R. Birjega, Hierarchical nanostructures of ZnO obtained in the presence of water soluble polymers, Powder Technol., 2013, 239, 56–58 CrossRef CAS PubMed.
- T. Wu, L. Y. Wang, Y. Zhang and M. S. Pei, Electrochemical synthesis of poly(3-thiophene acetic acid) nanowires with water-soluble macromolecule templates, RSC Adv., 2015, 5, 16684–16690 RSC.
- L. Wang, L. Y. Wang, H. L. Wang, Y. Zhang and M. S. Pei, High Yield of Ordered and Straight Polypyrrole Microwires Synthesized through a (Hydroxyethyl)cellulose Template, Chem. Lett., 2012, 41, 1692–1693 CrossRef CAS.
- L. Q. Li, P. Gao and K. C. Schuermann, Controllable Growth and Field-Effect Property of Monolayer to Multilayer Microstripes of an Organic Semiconductor, J. Am. Chem. Soc., 2010, 132, 8807–8809 CrossRef CAS PubMed.
- F. M. Ye, C. C. Cui and A. Kirkeminde, et al., Fluorescence Spectroscopy Studies of Silica Film Polarity Gradients Prepared by Infusion-Withdrawal Dip-Coating, Chem. Mater., 2010, 22, 2970–2977 CrossRef CAS.
- F. Marco, C. Aldo, V. Gaspare and G. David, Highly Controlled Dip-Coating Deposition of fct FePt Nanoparticles from Layered Salt Precursor into Nanostructured Thin Films: An Easy Way To Tune Magnetic and Optical Properties, Chem. Mater., 2012, 24, 1072–1079 CrossRef.
- Z. C. Hu, J. J. Xu, Y. Tian and Y. Z. Xian, Layer-by-layer assembly of polyaniline nanofibers/poly(acrylic acid) multilayer film and electrochemical sensing, Electrochim. Acta, 2009, 54, 4056–4061 CrossRef CAS PubMed.
- J. Stejskal, I. Sapurina and M. Trchova, Polyaniline nanostructures and the role of aniline oligomers in their formation, Prog. Polym. Sci., 2010, 35, 1420–1481 CrossRef CAS PubMed.
- X. Y. Zhang, W. J. Goux and S. K. Manohar, Synthesis of polyaniline nanofibers by “nanofiber seeding”, J. Am. Chem. Soc., 2004, 126, 4502–4503 CrossRef CAS PubMed.
- X. Y. Zhang, S. P. Surwade, V. Dua and R. Bouldin, Parent polythiophene nanofibers, Chem. Lett., 2008, 37, 526–527 CrossRef CAS.
- X. Y. Zhang, H. S. Kolla, X. H. Wang and K. Raja, Fibrillar growth in polyaniline, Adv. Funct. Mater., 2006, 16, 1145–1152 CrossRef CAS PubMed.
- X. Y. Zhang, A. G. MacDiarmid and S. K. Manohar, Chemical synthesis of PEDOT nanofibers, Chem. Commun., 2005, 5328–5330 RSC.
- X. Y. Zhang and S. K. Manohar, Bulk synthesis of polypyrrole nanofibers by a seeding approach, J. Am. Chem. Soc., 2004, 126, 12714–12715 CrossRef CAS PubMed.
- M. M. Ayad, N. A. Salahuddin, I. M. Minisy and W. A. Amer, Chitosan/polyaniline nanofibers coating on the quartz crystal microbalance electrode for gas sensing, Sens. Actuators, B, 2014, 202, 144–153 CrossRef CAS PubMed.
- Q. Q. Tan, Y. X. Xu, J. Yang and Y. Chen, Preparation and electrochemical properties of the ternary nanocomposite of polyaniline/activated carbon/TiO2 nanowires for supercapacitors, Electrochim. Acta, 2013, 88, 526–529 CrossRef CAS PubMed.
- X. Ma, M. Wang, G. Li, H. Chen and R. Bai, Preparation of polyaniline-TiO composite film with in situ polymerization approach and its gas-sensitivity at room temperature, Mater. Chem. Phys., 2006, 98, 241–247 CrossRef CAS PubMed.
- A. T. Ramaprasad, V. Rao, G. Sanjeev, S. P. Ramanani and S. Sabharwal, Grafting of polyaniline onto the radiation crosslinked chitosan, Synth. Met., 2009, 159, 1983–1990 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |