Electrical and magnetic properties of the pulsed laser deposited Ca doped LaMnO3 thin films on Si (100) and their electronic structures
Abstract
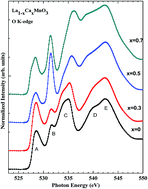
We report the effect of Ca doping on the structural, electrical, magnetic and electronic properties of stoichiometric La1−xCaxMnO3 (x = 0, 0.3, 0.5 and 0.7) (LCMO) thin films grown on Si (100) using a pulsed laser deposition technique. All these films exhibit a single-phase orthorhombic structure with the space group Pnma. Physical properties, such as surface roughness, grain size, Curie temperature ‘Tc’, activation energy ‘Ea’ and magneto-resistance, were studied as a function of Ca doping. These properties were correlated with the variation of the observed electrical transport and magnetic properties and their electronic structures. The electronic structures of these films were studied by X-ray absorption spectroscopy (XAS) at O K and at Mn L3,2-edges, which indicate an admixture of Mn2+, Mn3+, and Mn4+ ions and also an increase in the density of states with Ca doping. These mixed valence states of Mn ions in LCMO arise due to the doping of Ca in the La sites, which modifies the electrical and magnetic properties.


 Please wait while we load your content...
Please wait while we load your content...