Extraction of Ce(iv) from sulphuric acid solution by emulsion liquid membrane using D2EHPA as carrier
Abstract
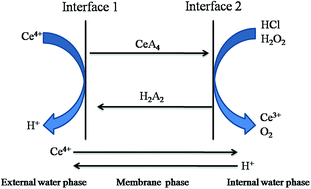
In order to provide a potential method for extracting Ce(IV), the extraction of Ce(IV) from sulphuric acid solution by an emulsion liquid membrane using D2EHPA as a carrier was investigated. The ELM system consisted of sulfonated kerosene as diluent, Span 80 as surfactant, liquid paraffin as intensifier and hydrochloric acid containing hydrogen peroxide as the inner aqueous solution. The influences of various parameters on the extraction of Ce(IV) were investigated. The optimum conditions for Ce(IV) extraction can be summarized as follows: D2EHPA concentration, 12% (v/v); Span 80 concentration, 2–3% (v/v); liquid paraffin concentration, 2–4% (v/v); hydrochloric acid concentration in the internal phase, 4–5 mol l−1; hydrogen peroxide concentration, 0.02 mol l−1; volume ratio of membrane phase to internal phase (Roi), 1.5; external phase acidity, 0.4–0.5 mol l−1; volume ratio of external phase to membrane phase (Rwe), 2; extraction time, 15 min; and stirring speed, 250 rpm. Experiments in which Ce(IV) was separated from RE(III) were then carried out under the optimum conditions, and the results indicated that the system is extremely selective for Ce(IV). The mechanism of Ce(IV) extraction has also been discussed. The loss for the experimental process was within 3%. The results reveal that the ELM method is a clean and cost-effective process for the extraction of Ce(IV) from sulphuric acid solution.

 Please wait while we load your content...
Please wait while we load your content...