Chemiluminescence determination of human serum albumin based on Co2+-catalyzed 2-(4-tert-butylphenyl)-4,5-di(2-furyl) imidazole/H2O2 system
Jing Kangab,
Jimin Shen*a,
Zhonglin Chena,
Jun Nana,
Xiao Huanga,
Lu Hanb and
Weiping Haoc
aState Key Laboratory of Urban Water Resource and Environment, School of Municipal and Environmental Engineering, Harbin Institute of Technology, Harbin 150090, P. R. China. E-mail: shenjimin@hit.edu.cn; Fax: +86-451-86283028; Tel: +86-451-86287000
bCollege of Chemistry, Jilin University, Changchun 130012, P. R. China
cHarbin Electric Machinery Company Limited, Harbin 150040, P. R. China
First published on 15th October 2015
Abstract
In this paper, the chemiluminescence (CL) reaction of 2-(4-tert-butylphenyl)-4,5-di(2-furyl) imidazole (t-BDFI) and H2O2 was investigated in alkaline medium. The H2O2 could directly oxidize t-BDFI to produce CL emission. The effects of some metal ions on CL of the t-BDFI/H2O2 system were examined. The results indicated that the addition of Co2+ into the t-BDFI/H2O2 CL system could induce significant enhancement of the CL signal. A CL system of t-BDFI/H2O2/Co2+ was developed. The experiment on the effect of human serum albumin (HSA) on the t-BDFI/H2O2/Co2+ CL system was carried out, and the result indicated that HSA could effectively inhibit the CL signal of this reaction. The inhibited CL intensity is linearly related to the logarithm of concentration of HSA. The linear range of the calibration curve is 5.0 × 10−8 to 1.0 × 10−6 mol L−1, and the corresponding detection limit (3σ) is 2.1 × 10−8 mol L−1. Based on this study, a novel CL method has been developed for the determination of HSA with high sensitivity and good selectivity. The proposed method has been successfully applied to the determination of HSA in human serum samples. This may intrigue researchers into gaining a new interest in investigating the CL property of heterocyclic imidazole derivatives.
Introduction
Human serum albumin (HSA) synthesized in the liver is the most abundant protein constituent of human blood plasma.1,2 The amount of this protein in the blood is approximately 35–58 mg mL−1, representing 55–65% of the total protein content.3 It is made up of 585 amino acids, and its polypeptide chain folds into a heart-shaped molecule.4–6 It plays an important role in the transportation and distribution of various exogenous and endogenous ligands, such as bilirubin, amino acids, nutrients, metal ions, fatty acids, and drugs etc.7–10 The distributions and metabolisms of small molecules in the body depend on the binding degree to HSA. The main physiology effect of HSA is to maintain the blood pH and colloid osmotic blood pressure. Moreover, HSA performs other functions, such as sequestering oxygen free radicals and inactivating various toxic lipophilic metabolites.11 The content of HSA can provide information for the diagnosis of diseases, and is often used as a reference for the determination of other components in biological samples. Therefore, the quantitative analysis of HSA is greatly important in biochemistry and clinical diagnosis.The most widely used chemical methods to determine HSA are those based on binding of protein with the dyes such as bromcresol green (BCG),12 coomassie brilliant blue (CBB),13 and bromophenol blue.14 Many of these methods have high reagent blank absorption, some of which could be reduced by addition of some detergent or heating, but it is laborious. Several analytical techniques, such as spectrophotometric,15,16 spectrofluorimetric,17 resonance light scattering,18 and electrochemical methods,19 have been reported for the determination of HSA. However, these methods have some limitations for determination of HSA, such as a narrow linear range, few interferents and slow reaction, which influenced the analysis of practical sample. It has been reported that chemiluminescence (CL) method was used to determine HSA.20,21 Owing to its low detection limit, wide dynamic range, rapid response, simple instrumentation and no background scattering light interference, the CL detection method is growing important in analytical chemistry,22,23 particularly in pharmaceutical and biomedical analysis.24,25
Heterocyclic imidazole derivatives have attracted increasing attention because of their potential applications in chemistry and materials science.26,27 These compounds play a very important role as mediators for synthetic reactions, primarily as a means for preparing functionalized materials.28 An important imidazole derivative is lophine (2,4,5-triphenylimidazole). It is a well-known potential CL compound since 1877, and has been used for analysis of some metal ions,29,30 and chlorinated compounds.31 Phenylimidazoles have been studied because of their important laser properties.32 Further substitution by phenyl groups has been studied for other significant optical properties. Recently, a number of lophine derivatives were synthesized based on lophine skeleton substituted at the ortho- , meta- and para-substituted in the 2-phenyl ring and para-substituted in the 4- and 5-aryl rings according to slightly modified procedure of the Debus method.33,34 A variety of lophine analogues having 2-pyridyl or 2-furyl group at both 4- and 5-positions of heterocyclic imidazole derivatives have been reported.35,36 Heterocyclic imidazole derivatives are a new type of luminescent material with unique optical properties, and have been studied with regard to their ultraviolet (UV), photoluminescence (PL) and CL properties.36,37
Recently, we have synthesized a variety of imidazole derivatives, and evaluated their CL properties with the aim of developing new CL reagents and reactions.38–40 In the present work, 2-(4-tert-butylphenyl)-4,5-di(2-furyl) imidazole (t-BDFI) was synthesized according to the reported method.40 The H2O2 could directly oxidize t-BDFI to produce CL emission in alkaline medium. The addition of Co2+ into the t-BDFI/H2O2 CL system could induce significant enhancement of CL signal. The possible enhancement mechanism of the CL reaction was also further investigated. The effects of experimental conditions were investigated. Under the optimal conditions, HSA could induce inhibition of CL signal of the t-BDFI/H2O2/Co2+ system. Based on the inhibition of CL for the t-BDFI/H2O2/Co2+ system by HSA, a sensitive CL method has been developed for the determination of HSA. The proposed method was applied to the determination of HSA in human serum samples. The human serum samples were prepared by the salting-out method with saturated ammonium sulfate solution as precipitating agent,20,41 and the obtained results were satisfactory.
Experimental
Reagents and materials
All the reagents were of analytical reagent grade and all solutions were prepared with ultra-pure water. CoCl2·6H2O was purchased from Shanghai Chemical Reagent Co. Ammonium acetate, acetic acid, ammonium sulfate, NaOH, KCl, NaCl, CaCl2, Ni(NO3)2·6H2O, BaCl2·2H2O, CdCl2, MnSO4·H2O, Cr(NO3)3·9H2O, Pb(NO3)2, FeCl2, FeCl3, ethanol and H2O2 (30%) were purchased from Beijing Chemical Plant in China. HSA was purchased from Sigma. L-Asparagine, L-tryptophan, DL-β-phenylalanine and L-tyrosine were purchased from Beijing Dingguo Biotechnology Co., Ltd. Furfural and 4-tert-butylbenzaldehyde were purchased from Tianjin Guangfu Fine Chemical Research Institute.The 1.0 × 10−3 mol L−1 stock solution of t-BDFI was prepared by dissolving 8.5 mg of t-BDFI in 25 mL ethanol, and the working solution (2.5 × 10−4 mol L−1) was prepared by diluting the stock solution with ultra-pure water.
The 1.0 × 10−3 mol L−1 stock solution of Co2+, Cr3+, Pb2+ etc. were prepared by dissolving 11.9 mg of CoCl2·6H2O, 20.0 mg of Cr(NO3)3·9H2O and 16.6 mg of Pb(NO3)2 etc. in 50 mL ultra-pure water, respectively. And the working solution (1.0 × 10−7 mol L−1) were prepared by diluting the stock solution with ultra-pure water.
Human serum samples were obtained from 4 patients in Qianwei Hospital of Jilin University. All experiments were performed in compliance with the relevant laws and institutional guidelines, and the institutional committees have approved the experiments. The informed consent was obtained for any experimentation with human subjects.
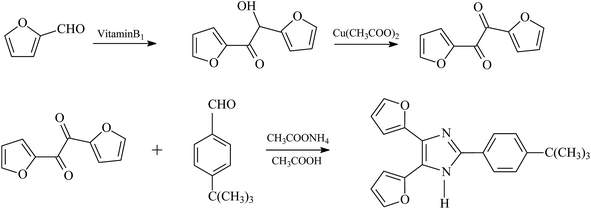
Synthesis and characterization of t-BDFI
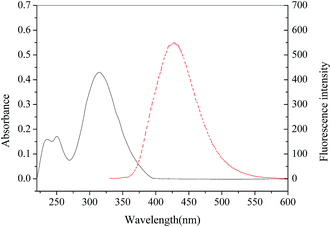
The t-BDFI was synthesized according to the literature.40 The synthesis route is shown in Fig. 1. First, furil was synthesized from furfural by benzoic condensation and oxidation, and then the t-BDFI was prepared by the condensation reaction of furil with 4-tert-butylbenzaldehyde, ammonium acetate and acetic acid. The absorption and fluorescence spectra of the t-BDFI were investigated, and the results are shown in Fig. 2. The first excitonic absorption peak of the t-BDFI is at around 316 nm, corresponding with the fluorescence peak of 427 nm.The t-BDFI was characterized by melting point, IR, MS and NMR. The obtained results by elemental analysis were in conformity with the theoretical results. The results are described as follows: mp 189–191 °C. IR (KBr), ν (cm−1): 3122, 3068, 2962, 2860, 1602, 1528, 1491, 1436, 1364, 1268, 1201, 1080, 971, 886, 839, 732, 593. 1H NMR (300 MHz, CDCl3), δ (ppm): 7.85 (d, J = 8.5 Hz, 2H), 7.49 (dd, J = 1.8, 0.7 Hz, 2H), 7.46 (d, J = 8.6 Hz, 2H), 7.00 (d, J = 3.3 Hz, 2H), 6.52 (dd, J = 3.4, 1.8 Hz, 2H), 1.34 (s, 9H) ppm. 13C NMR (126 MHz, CDCl3), δ (ppm): 152.89, 146.69, 126.81, 126.22, 125.67, 112.16, 107.91, 35.19, 31.62. MS (m/z): (M + H)+ 334.2 (calcd. 332.40). Calcd for C21H20N2O2: C, 75.88; H, 6.06; N, 8.43. The elemental analysis gave the molecular formula of C21H20N2O2 (found: C, 75.91; H, 6.24; N, 8.67).
Apparatus
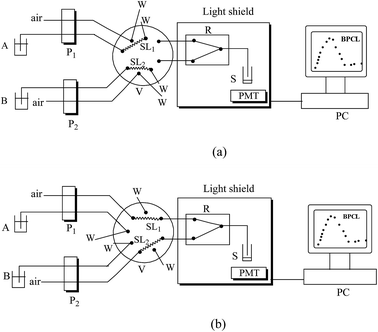
The CL analysis was conducted on a laboratory-built steady injection CL system. The schematic diagram of the system is shown in Fig. 3. The steady injection Analysis Processor FIA-3100 (Beijing Wantuo Instruments Co., Ltd.) consists of two peristaltic pumps, a sixteen-hole eight-way valve and a digital-system to control the time and pump pressure. The polytetrafluoroethylene (PTFE) tube (0.8 mm i.d.) was used as the connection pipe in the steady system. The CL emission was detected by an ultra-weak luminescence analyzer (type BPCL manufactured at the Institute of Biophysics, Chinese Academy of Sciences, Beijing, China).The PL spectra were recorded on a RF-5301 spectrofluorimeter (Shimadzu, Japan). The absorption spectra were recorded on an Australian GBC Cintra 10e UV-vis spectrometer within the wavelength range from 200 to 800 nm. The CL spectrum was obtained with a series of interference filters. The interference filters were inserted between the sample cell and the photomultiplier tube (PMT). The spectral range detected with the PMT is from 400 to 640 nm.
General procedure for CL analysis
The experimental results were obtained by using the following operation parameters: sample loop volume, 200 μL; sampling time, 13 s; sample injection time, 13 s; the PMT negative voltage, −1000 V; the integral time of the CL signal, 60 s. 200 μL of t-BDFI, 100 μL of Co2+ and 100 μL of HSA sample solutions were first added into the sample cell S (optical glass tube 1 cm i.d.), and then H2O2 and NaOH solutions were simultaneously injected into the S with the steady injection system.The operation mode is as follows: in sequence 1 (Fig. 3a), pumps P1 and P2 were activated, and valve V was in the loading position. P1 was used to deliver H2O2 solution into the sample loop1 (SL1) and P2 was used to deliver NaOH solution into the sample loop2 (SL2). In sequence 2 (Fig. 3b), P1 and P2 were activated, and V was in the injection position. P1 and P2 were used to deliver the air current. The H2O2 and NaOH solutions were simultaneously pumped into chemifold R where they were mixed. The mixed solution was carried into S and reacted with the mixture of t-BDFI, Co2+ and HSA sample solutions. The CL signal was measured and recorded. After determination, the mixed solution was emptied and S was washed and dried. All the experiment procedures were performed in triplicate.
The concentration of analyte was quantified by measuring the inhibited CL intensity, ΔI = I0 − Is, where I0 and Is are CL signals in the absence and presence of HSA, respectively.
Preparation of sample
10 mL of human blood was at rest for 10 minutes, and then centrifugalized for 20 minutes at 1000 rpm. The upper human serum solution was added into a flask, and the lower hemocyte sediment was removed. 300 μL of human serum sample and an equal volume of normal saline (NS) were mixed, and added into a 2 mL centrifuge tube. 600 μL of saturated ammonium sulfate solution was added drop-wise into the centrifuge tube when the solution was stirred continuously. The solution was at rest for 1 hour, and then centrifugalized for 30 minutes at 4000 rpm. The upper HSA solution was added into a flask, and the lower sediment was dissolved in 600 μL of NS. 300 μL of saturated ammonium sulfate solution was added drop-wise into the resulted solution when the solution was stirred continuously. The solution was centrifugalized for 30 minutes at 4000 rpm. Then the upper HSA solution obtained was also added into the flask, and the lower globulin and fibrin sediment was removed. To purify the solution in the flask, the free ions were removed via dialysis for 24 hours in water (4 °C). The resulting solution was diluted to 25 mL volumetric flask with ultra-pure water, and diluted 25-fold for analysis.Results and discussion
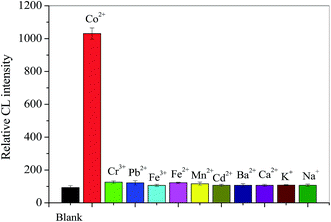
Effects of metal ions on CL of t-BDFI/H2O2 system
The CL reaction between t-BDFI and H2O2 was further investigated. The H2O2 could directly oxidize t-BDFI to produce CL emission in an alkaline solution. The effects of some metal ions, Co2+, Cr2+, Pb2+, Fe3+, Fe2+, Mn2+, Cd2+, et al., on the CL of t-BDFI/H2O2 system were examined. The results are shown in Fig. 4. It can be seen from Fig. 4 that the addition of Co2+ into the t-BDFI/H2O2 system could induce significant enhancement of CL signal. Co2+ that causes the most intense light emission are fairly strong oxidants.42 The result might be related to outer electron structure of metal ion. So Co2+ was chosen to enhance the CL intensity of the t-BDFI/H2O2 system in the further experiment.Effect of HSA on the CL of t-BDFI/H2O2/Co2+ system
Fig. 5 shows the dynamic CL intensity–time profiles of the t-BDFI/H2O2, t-BDFI/H2O2/Co2+, and t-BDFI/H2O2/Co2+/HSA systems. It was found that the addition of HSA into the t-BDFI/H2O2/Co2+ system could induce inhibition of CL signal. Based on the inhibition effect, a sensitive CL method has been developed for the determination of the HSA.Optimization of the reaction conditions
The proposed CL reaction in alkaline medium can produce strong CL signal. The NaOH solution was employed as the reaction medium, and the effect of the concentration of NaOH on the CL intensity was further examined in the range of 0.01–1.0 mol L−1. The experimental results are shown in Fig. 6a. It can be seen from Fig. 6a that the CL intensity increases when the NaOH concentration increases from 0.01 to 0.25 mol L−1, and the intensity decreases when the NaOH concentration is higher than 0.25 mol L−1. Hence, the NaOH solution of 0.25 mol L−1 was chosen as the reaction medium.The influence of H2O2 concentration on the CL intensity was studied in the range of 0.001–1.0 mol L−1, and the results are shown in Fig. 6b. The CL intensity increases with increasing H2O2 concentration up to 0.05 mol L−1. When the H2O2 concentration is higher than 0.05 mol L−1, the CL intensity of the system decreases with the increasing concentration of H2O2. The H2O2 concentration of 0.05 mol L−1 was chosen for the further research.
The effect of t-BDFI concentration on the CL intensity of the studied system was tested, as shown in Fig. 6c. The CL intensity increases with the increasing concentration of t-BDFI from 1.0 × 10−6 to 2.5 × 10−4 mol L−1. When the t-BDFI concentration is higher than 2.5 × 10−4 mol L−1, the CL intensity is slowly increased. Therefore, the t-BDFI solution of 2.5 × 10−4 mol L−1 was chosen for subsequent studies.
The effect of Co2+ concentration on the CL intensity was tested, and the experimental results are shown in Fig. 6d. The concentration of the Co2+ has great influence on the CL intensity. When the concentration of Co2+ is lower than 1.0 × 10−6 mol L−1, the CL intensity increases with the increase of Co2+ concentration, and the intensity slowly decreases when the Co2+ concentration is higher than 1.0 × 10−6 mol L−1. Therefore, the optimum concentration of Co2+ was chosen to be 1.0 × 10−6 mol L−1.
There is a good linear relationship between the enhanced CL intensity and the logarithm of Co2+ concentration in the range of low concentration. The results are shown in Fig. 6d inset. The linear range is 5.0 × 10−9 to 2.5 × 10−7 mol L−1, linear regression equation is ΔI = 208![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450
450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C − 126
C − 126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 (C, nmol L−1; r = 0.9995), and the detection limit is 1.1 × 10−9 mol L−1. The results demonstrate that the proposed CL system may be used to detect Co2+.
250 (C, nmol L−1; r = 0.9995), and the detection limit is 1.1 × 10−9 mol L−1. The results demonstrate that the proposed CL system may be used to detect Co2+.
Analytical applications
Under the optimized conditions, the linear relationship between the inhibited CL intensity and the logarithm of HSA concentration was obtained. The experimental results are shown in Fig. 7. The linear range is 5.0 × 10−8 to 1.0 × 10−6 mol L−1, linear regression equation is ΔI = 514![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 219
219![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C − 865
C − 865![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 818 (C, nmol L−1; r = 0.9952) and the detection limit is 2.1 × 10−8 mol L−1. The relative standard deviation for determination of 4.0 × 10−7 mol L−1 HSA is 5.1% (n = 9).
818 (C, nmol L−1; r = 0.9952) and the detection limit is 2.1 × 10−8 mol L−1. The relative standard deviation for determination of 4.0 × 10−7 mol L−1 HSA is 5.1% (n = 9).
In order to assess the selectivity of the proposed method, the interference of some foreign substances was tested when the concentration of HSA was 5.0 × 10−7 mol L−1. The tolerance level was defined as the amount of foreign species that produce an error not exceeding ±5% in the determination of the analyte. The results showed that no interference could be observed for 1000-fold Na+, K+, Ba2+, Ca2+, Pb2+, NH4+, Cl−, NO3−, L-asparagine, L-tryptophan, 100-fold DL-β-phenylalanine, 50-fold Cd2+, 20-fold Mn2+, L-tyrosine, and 1-flod Cr3+.
The proposed method was applied to the determination of HSA in human serum samples, and the human serum samples were prepared by the salting-out method. Salting out is a method used to separate classes of proteins.43,44 The solubility of proteins in aqueous solution depends on the distribution of hydrophilic and hydrophobic amino acid residues on the protein surface. After protein folding in aqueous solution, hydrophobic amino acids usually form protected hydrophobic areas while hydrophilic amino acids interact with the molecules of solvation and allow proteins to form hydrogen bonds with the surrounding water molecules. If enough of the protein surface is hydrophilic, the protein can be dissolved in water. When the salt concentration is increased, some of the water molecules are attracted by the salt ions, which decreases the number of water molecules available to interact with the charged part of the protein. As a result of the increased demand for solvent molecules, the protein–protein interactions are stronger than the solvent–solute interactions; the protein molecules coagulate by forming hydrophobic interactions with each other. As different proteins have different compositions of amino acids, different protein molecules precipitate at different concentrations of salt solution. The type of salt being used and the concentration of the salt can be varied to selectively precipitate protein. Unwanted proteins can be removed from a protein solution mixture by salting out as long as the solubility of the protein in various concentrations of salt solution is known, and the precipitate can be removed by filtration or centrifugation.
Ammonium sulfate is the most widely used as precipitant to induce salting-out. It is reported, that fibrin is totally precipitated when adding saturated ammonium sulfate solution to the serum at up to 20% volume fraction, globulin is totally precipitated when adding saturated ammonium sulfate solution to the serum at up to 50% volume fraction, and HSA is not precipitated when adding saturated ammonium sulfate solution to the serum at less than 50% volume fraction.20,44 In this work, the HSA was separated from globulin and fibrin in human serum by using the salting-out method with about 50% volume fraction of saturated ammonium sulfate solution as precipitating agent. The results are shown in Table 1. It can be seen from Table 1 that the results obtained were found to be in agreement with the commonly used BCG method. The relative errors for the analytical results were from −2.1% to 4.3%. Therefore the developed method can be easily performed and affords good precision and accuracy when applied to real samples.
| Sample | Bromocresol green method (g L−1) | Proposed method (g L−1) | RSD (%) | Relative error (%) |
|---|---|---|---|---|
| 1 | 47 | 49 | 4.3 | 4.3 |
| 2 | 50 | 52 | 5.2 | 4.0 |
| 3 | 46 | 46 | 3.7 | 0.0 |
| 4 | 48 | 47 | 4.6 | −2.1 |
Possible CL reaction mechanism
To explain the CL reaction mechanism and confirm the emission species, the following experiments were performed. The CL spectra of t-BDFI/H2O2, t-BDFI/H2O2/Co2+, and t-BDFI/H2O2/Co2+/HSA systems were examined, as shown in Fig. 8. The results showed that the maximum emission appeared at 535 nm for the three reaction systems. When the Co2+ was added into the t-BDFI/H2O2 system, the shape of the CL emission peak was not significantly affected, but the CL intensity was significantly enhanced. The CL intensity decreased when HSA was added into the t-BDFI/H2O2/Co2+ system. The results indicate that the Co2+ and HSA are only enhancement or inhibition reagents and do not change the mechanism of the t-BDFI/H2O2 CL reaction.From Fig. 2 it can be seen that the characteristic emission peak of t-BDFI was at 427 nm. The PL spectra did not coincide with the CL spectra of t-BDFI. The results revealed that the luminophor of t-BDFI/H2O2 system is not t-BDFI but the intermediate oxidation product of t-BDFI. The possible oxidized CL mechanism is similar to that of lophine, which is clarified that a hydroperoxide as a reaction intermediate is intramolecularly decomposed to yield an excited singlet state of diaroylamidine, followed by the emission of light.28
The possible mechanism of CL emission is shown in Fig. 9. In this reaction, H2O2 reacts with dissolved oxygen to form hydroxyl radical (˙OH), and the Co2+ catalyze the decomposition of H2O2 and production of ˙OH and hydroperoxyl radical (HO2˙).31 The produced ˙OH and HO2˙ can be converted into superoxide radical (˙O2−) in the presence of strong base and H2O2.45 The produced ˙O2− reacts with t-BDFI to form the peroxide excited state of diaroylamidine via a dioxetane structure.28 The CL emission is produced when the peroxide excited state of diaroylamidine returns to the ground state. Moreover, a t-BDFI–Co2+ complex could be formed,42 and it changes molecular electron delocalization of t-BDFI, which catalyzes the reaction between ˙O2− and t-BDFI. When HSA chelates with Co2+,46,47 which inhibits the catalytical effect of Co2+ on CL of the t-BDFI/H2O2 system.
Conclusion
This work is concerned with the CL of t-BDFI. The H2O2 could directly oxidize t-BDFI to produce CL emission in an alkaline solution. The effects of various metal ions on the t-BDFI/H2O2 CL system were investigated, and the results indicated that Co2+ could effectively enhance the sensitivity of the CL reaction. Based on this study, t-BDFI/H2O2/Co2+ CL system was developed. The addition of HSA into the t-BDFI/H2O2/Co2+ CL system could induce significant inhibition of CL signal. The inhibited CL intensity was linearly related to the logarithm of HSA concentration in the certain concentration range. The CL system was successfully applied to the determination of HSA in human serum samples, and the results were in agreement with those obtained by BCG method. By comparison with some existing methods,17–20 the proposed method has advantages of high selectivity and sensitivity, instrumental simplicity, low cost and wide linear response range for determination of HSA. The work would further enable researchers to exploit more CL analytical applications of the heterocyclic imidazole derivatives.Acknowledgements
The work was supported by Open Project of State Key Laboratory of Urban Water Resource and Environment (Harbin Institute of Technology) (No. ES201311), the Fundamental Research Funds for the Central Universities (No. HIT. NSRIF. 2015091), and China Postdoctoral Science Foundation (No. 2013M541395).References
- F. Cui, Y. Yan, Q. Zhang, X. Yao, G. Qu and Y. Lu, Spectrochim. Acta, Part A, 2009, 74, 964–971 CrossRef PubMed.
- Y. Ni, S. Su and S. Kokot, Spectrochim. Acta, Part A, 2010, 75, 547–552 CrossRef PubMed.
- N. T. Deftereos, N. Grekas and A. C. Calokerinos, Anal. Chim. Acta, 2000, 403, 137–143 CrossRef CAS.
- X. M. He and D. C. Carter, Nature, 1992, 358, 209–215 CrossRef CAS PubMed.
- N. Shahabadi, A. Khorshidi and N. H. Moghadam, Spectrochim. Acta, Part A, 2013, 114, 627–632 CrossRef CAS PubMed.
- S. Tabassum, W. M. Al-Asbahy, M. Afzal and F. Arjmand, J. Photochem. Photobiol., B, 2012, 114, 132–139 CrossRef CAS PubMed.
- D. C. Carter and J. X. Ho, Adv. Protein Chem., 1994, 45, 153–203 CrossRef CAS.
- X. Pan, P. Qin, R. Liu and J. Wang, J. Agric. Food Chem., 2011, 59, 6650–6656 CrossRef CAS PubMed.
- S. R. Feroz, S. B. Mohamad, N. Bujang, S. N. Malek and S. Tayyab, J. Agric. Food Chem., 2012, 60, 5899–5908 CrossRef CAS PubMed.
- U. Kragh-Hansen, V. T. Chuang and M. Otagiri, Biol. Pharm. Bull., 2002, 25, 695–704 CAS.
- S. Tunç, O. Duman, İ. Soylu and B. K. Bozoğlan, J. Lumin., 2014, 151, 22–28 CrossRef PubMed.
- F. L. Rodkey, Clin. Chem., 1965, 11, 478–487 CAS.
- T. Zor and Z. Selinger, Anal. Biochem., 1996, 236, 302–308 CrossRef CAS PubMed.
- R. Flores, Anal. Biochem., 1978, 88, 605–611 CrossRef CAS.
- L. F. Capitán-Vallvey, O. Duque, G. Mirón-García and R. Checa-Moreno, Anal. Chim. Acta, 2001, 433, 155–163 CrossRef.
- H. W. Gao, J. Jiang and L. Q. Yu, Analyst, 2001, 126, 528–533 RSC.
- C. Jiang and L. Luo, Anal. Chim. Acta, 2004, 506, 171–175 CrossRef CAS PubMed.
- L. Dong, Y. Li, Y. Zhang, X. Chen and Z. Hu, Spectrochim. Acta, Part A, 2007, 66, 1317–1322 CrossRef PubMed.
- W. Sun, X. Wang, K. Jiao and L. Lu, Chin. J. Anal. Chem., 2005, 1, 143 Search PubMed.
- N. T. Deftereos, N. Grekas and A. C. Calokerinos, Anal. Chim. Acta, 2000, 403, 137–143 CrossRef CAS.
- W. Xu, Y. Wei, D. Xing and Q. Chen, Anal. Sci., 2008, 24, 115–119 CrossRef CAS.
- C. A. Marquette and L. J. Blum, Anal. Bioanal. Chem., 2006, 385, 546–554 CrossRef CAS PubMed.
- K. A. Fletcher, S. O. Fakayode, M. Lowry, S. A. Tucker, S. L. Neal, I. W. Kimaru, M. E. McCarroll, G. Patonay, P. B. Oldham, O. Rusin, R. M. Strongin and I. M. Warner, Anal. Chem., 2006, 78, 4047–4068 CrossRef CAS PubMed.
- J. Lin and H. Ju, Biosens. Bioelectron., 2005, 20, 1461–1470 CrossRef CAS PubMed.
- J. Yakovleva, R. Davidsson, A. Lobanova, M. Bengtsson, S. Eremin, T. Laurell and J. Emnéus, Anal. Chem., 2002, 74, 2994–3004 CrossRef CAS.
- N. Fridman, S. Speiser and M. Kaftory, Cryst. Growth Des., 2006, 6, 1653–1662 CAS.
- J. Santos, E. A. Mintz, O. Zehnder, C. Bosshard, X. R. Bua and P. Günterb, Tetrahedron Lett., 2001, 42, 805–808 CrossRef CAS.
- K. Nashima, Biomed. Chromatogr., 2003, 17, 83–95 CrossRef PubMed.
- D. F. Marho and J. D. Ingle Jr, Anal. Chem., 1981, 53, 294–298 CrossRef.
- K. Nakashima, H. Yamasaki, R. Shimoda, N. Kuroda, S. Akiyama and W. R. G. Baeyens, Biomed. Chromatogr., 1997, 11, 63–64 CrossRef CAS.
- A. MacDonald, K. W. Chan and T. A. Nieman, Anal. Chem., 1979, 51, 2077–2082 CrossRef CAS.
- A. C. Testa, Spectrochim. Acta, Part A, 2000, 56, 901–904 CrossRef CAS.
- N. Fridman, M. Kaftory and S. Speiser, Sens. Actuators, B, 2007, 126, 107–115 CrossRef CAS PubMed.
- K. Nakashima, H. Yamasaki, N. Kuroda and S. Akiyama, Anal. Chim. Acta, 1995, 303, 103–107 CrossRef CAS.
- S. A. Siddiqui, U. C. Narkhede, S. S. Palimkar, T. Daniel, R. J. Lahoti and K. V. Srinivasan, Tetrahedron, 2005, 61, 3539–3546 CrossRef CAS PubMed.
- K. Nakashima, Y. Fukuzaki, R. Nomura, R. Shimoda, Y. Nakamura, N. Kuroda, S. Akiyama and K. Irgum, Dyes Pigm., 1998, 38, 127–136 CrossRef CAS.
- N. Fridman, M. Kaftory, Y. Eichen and S. Speiser, J. Mol. Struct., 2009, 917, 101–109 CrossRef CAS PubMed.
- J. Kang, Y. Zhang, L. Han, J. Tang, S. Wang and Y. Zhang, J. Photochem. Photobiol., A, 2011, 217, 376–382 CrossRef CAS PubMed.
- J. Kang, L. Han, Z. Chen, J. Shen, J. Nan and Y. Zhang, Food Chem., 2014, 159, 445–450 CrossRef CAS PubMed.
- B. Li, Q. Gu, Y. He, T. Zhao, S. Wang, J. Kang and Y. Zhang, C. R. Chim., 2012, 15, 784–792 CrossRef CAS PubMed.
- A. Kovacs and A. Guttman, Curr. Med. Chem., 2013, 20, 483–490 CAS.
- M. Allan, W. C. Kenneth and A. N. Timothy, Anal. Chem., 1979, 51, 2077–2082 CrossRef.
- F. Y. Yohko, U. Tomoya, T. Hajime, T. Yasuko and Y. Hironari, J. Phys. Chem. Lett., 2011, 2, 995–999 CrossRef.
- A. C. Dumetz, A. M. Snellinger-O'Brien, E. W. Kaler and A. M. Lenhoff, Protein Sci., 2007, 16, 1867–1877 CrossRef CAS PubMed.
- A. L. Rose and T. D. Waite, Anal. Chem., 2001, 73, 5909–5920 CrossRef CAS.
- M. Sokołowska, M. Wszelaka-Rylik, J. Poznański and W. Bal, J. Inorg. Biochem., 2009, 103, 1005–1013 CrossRef PubMed.
- D. Bar-Or, L. T. Rael, R. Bar-Or, D. S. Slone, C. W. Mains, N. K. Rao and C. G. Curtis, Clin. Chim. Acta, 2008, 387, 120–127 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |