Synthesis and characterization of flurbiprofen axetil-loaded electrospun MgAl-LDHs/poly(lactic-co-glycolic acid) composite nanofibers
Abstract
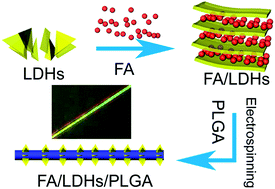
We have reported a facile method to fabricate drug-loaded hybrid nanofibers for drug sustained release. In our work, a model drug FA was intercalated into the interlayers of layered double hydroxides (LDHs) by ion-exchange intercalation. The particles were dispersed into the PLGA solution to form the electrospun hybrid nanofibers. The intercalation of FA into the LDHs interlayers (MgAl–FA-LDHs) and the composite nanofibers were characterized via different techniques. The results of XRD and FTIR indicate that FA molecules are intercalated into the MgAl-LDHs interlayers. The formed composite nanofibers exhibit a uniform and smooth morphology and the hydrophilicity did not improve significantly. Importantly, the drug-loaded MgAl–FA-LDHs/PLGA shows a sustained release profile which indicates the MgAl-LDHs can be candidates for drug sustained release.

 Please wait while we load your content...
Please wait while we load your content...