Microwave-assisted, ruthenium-catalyzed intramolecular amide-alkyne annulation for the rapid synthesis of fused tricyclic isoquinolinones†
Abstract
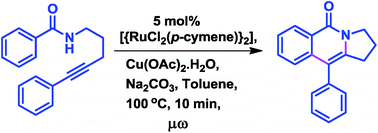
Microwave irradiation strongly accelerates the ruthenium(II)-catalyzed intramolecular annulation of alkyne appended benzamides to generate the fused tricyclic isoquinolinone scaffolds through a domino C–C and C–N bond formation by C–H and N–H activation, which is very useful for the synthesis of several analogues of tricyclic isoquinolinone alkaloids. The reaction proceeds with high functional group tolerance to furnish the products in good to high yields.

 Please wait while we load your content...
Please wait while we load your content...