Enhanced cycle life of lead-acid battery using graphene as a sulfation suppression additive in negative active material†
Abstract
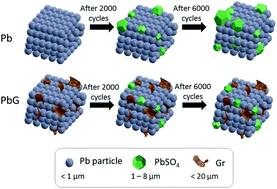
In this article, we report the addition of graphene (Gr) to negative active materials (NAM) of lead-acid batteries (LABs) for sulfation suppression and cycle-life extension. Our experimental results show that with an addition of only a fraction of a percent of Gr, the partial state of charge (PSoC) cycle life is significantly improved by more than 140% from 7078 to 17 157 cycles. The particle size on a charged Pb-graphene (PbG) plate after the PSoC test is also found to be reduced by around 25% when compare with a Pb plate. Charge and discharge densities measurements from the cyclic voltammetry (CV) test show an enhancement with the addition of Gr, indicating an improvement in the reversibility reaction of PbSO4. An electrochemical model, which takes into account of reduced interfacial resistance, improved charge transfer and enhanced electroactive surface area, is proposed to elucidate the role of Gr throughout the course of a PSoC cycle test. It is demonstrated that experimental results are aligned with our proposed model with enhanced cycle life performance, where PbG plate maintains a higher electroactive surface area for adsorption and desorption of Pb2+ ions at the interface between active material and electrolyte occurs in parallel to reduced charge transfer.

 Please wait while we load your content...
Please wait while we load your content...