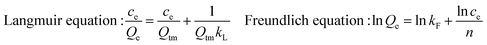β-Cyclodextrin/chitosan–magnetic graphene oxide–surface molecularly imprinted polymer nanocomplex coupled with chemiluminescence biosensing of bovine serum albumin
Huimin Duan,
Leilei Li,
Xiaojiao Wang,
Yanhui Wang,
Jianbo Li and
Chuannan Luo*
Key Laboratory of Chemical Sensing & Analysis in Universities of Shandong (University of Jinan), School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China. E-mail: chm_luocn@ujn.edu.cn; Tel: +86 0531 89736065
First published on 30th July 2015
Abstract
In this report, a sensitive and selective chemiluminescence (CL) biosensor for bovine serum albumin (BSA) coupled with a surface molecularly imprinted polymer nanocomplex using β-cyclodextrin/chitosan–magnetic graphene oxide as backbone material (β-CD/Cs–MGO–SMIP) was investigated. The material β-CD/Cs–MGO combined with β-cyclodextrin, chitosan and graphene oxide was used to provide multiple imprinting sites and a large surface area was characterized by SEM, XRD and FTIR. It was found that β-CD/Cs–MGO–SMIP followed the Langmuir isotherm equation and pseudo-second order sorption kinetics when binding the template. This material demonstrated fast mass transfer, a promoted rate of removal of the biomacromolecule and excellent recognition and adsorption ability for the imprinting cavities situated at the surface of β-CD/Cs–MGO, which enabled easy access to BSA. Subsequently, a highly sensitive CL biosensor for BSA was proposed based on the strong recognition effect between β-CD/Cs–MGO–SMIP and BSA which led to a high selectivity of the sensor, and the proposed biosensor could assay in the range 5.0 × 10−7 to 1.0 × 10−4 mg mL−1 with a detection limit of 1.1 × 10−7 mg mL−1. The obtained recoveries were between 94% and 106% when determining samples.
1 Introduction
Bovine serum albumin (BSA), thought to be a potential autoimmune trigger of insulin-dependent diabetes mellitus (IDDM) (though the causal association remains a controversial topic1), is a component of the whey protein system in cows' milk, bovine milk or milk-based paediatric formulae used during infancy.2 The level of BSA in bovine milk is used as a marker of the health of the mammary gland and of milk quality.3 Analytical approaches for the determination of bovine serum albumin that have been proposed include chemometrics,2 optical biosensors,3 etc. Cheap, convenient and sensitive chemiluminescence (CL)4 biosensors for selective determination of BSA have been intentionally developed, but specific molecular recognition ability is required. Thus, novel receptor-like techniques for biological recognition have emerged rapidly to selectively recognize BSA.For this purpose, the surface molecular imprinting technique was considered as a promising way to design a synthetic receptor in which the space structure of mimicking biomolecules was recorded and specific recognition was achieved.5 Certainly, the synthesis of surface molecular imprinting supporting materials that can improve the selectivity and adsorption capacity towards target biomacromolecules is particularly important.6
Recently, chitosan (Cs) has attracted considerable attention7 as one of the most promising materials due to its biodegradability, biocompatibility and non-toxicity.8 As a natural mucopolysaccharide with similar structural characteristics to cellulose, Cs has been applied in the preparation of molecularly imprinted resins,9 integrated-optical sensors10 and porous membranes.11 On account of its abundant hydroxyl and amino groups, when preparing surface molecularly imprinting polymers (SMIPs), Cs has the great advantage of multiple imprinting sites which can accelerate the imprinting process, and improve the selectivity and adsorption capacity.12
β-Cyclodextrin (β-CD), with a lipophilic inner cavity and hydrophilic outer surfaces which are able to interact with a large variety of guest molecules to form non-covalent inclusion complexes,13 has already demonstrated its potential in separation and analytical applications since it can bind Ångstrom-sized guests through apolar interactions in protic media.14 As a supramolecular host compound, β-CD has potential as a functional monomer to prepare MIPs to achieve high selectivity and great adsorption capacity.15
In the current century, as a fascinating new carbon material with a one-atom-thick honeycomb structure, graphene oxide (GO) has attracted worldwide attention.16 Due to its large specific surface area, and good biocompatibility and chemical stability, GO has been used as supporting material to prepare SMIPs.17 Moreover, due to abundant hydroxyl and carboxyl groups, integration of GO with other materials, such as organic functional materials, is highly desirable.18,19 In this case, a method to improve the properties of GO was the coprecipitation of Fe3O4 nanoparticles onto the surface of GO sheets to obtain magnetic graphene oxide (MGO) which combined the merits of GO and Fe3O4 nanoparticles of high adsorption capacity and easy separation, respectively.20,21
In particular, the combination of β-CD and Cs with MGO (β-CD/Cs–MGO) serving as supporting material in the preparation process of SMIPs made the recognition of the nanocomplex (β-CD/Cs–MGO–SMIP) towards BSA selective and efficient due to its numerous binding sites. Accordingly, fast mass transfer, a promoted rate of removal of the biomolecule and excellent recognition and adsorption ability of the imprinting cavities in proximity of the surface of β-CD/Cs–MGO was achieved, which enabled easy access to BSA. When the synthesized β-CD/Cs–MGO–SMIP, as a potential optical receptor, was introduced into the CL analytical method for the detection of biomacromolecular BSA, good analytical performance characteristics, such as selectivity and sensitivity, were obtained. Finally, the proposed β-CD/Cs–MGO–SMIP–CL biosensor was applied to detect BSA in samples.
2 Experimental
2.1 Materials
BSA (96%), N-N-methylene double acrylamide (MBA, A.R), N,N,N′,N′-tetramethylethylenediamine (TEMED, A.R) and diethyl amino ethyl methacrylate (DMAEMA, 99%) were purchased from Aladdin Industrial Co. (China); β-CD, Cs, ferrous sulfate (A.R) and ammonium persulphate (APS, AR) were supplied by Sinopharm Chemical Reagent Co. Ltd (China); ethanol, acetic acid, luminol and all other chemicals unless otherwise specified were of analytical reagent grade and were used without further purification.Redistilled water was used throughout the work. Phosphate buffer (PBS, pH = 7.4, 0.01 mol L−1) solution was used to prepare all BSA solutions, which were stored in a refrigerator (4 °C).
2.2 Apparatus
An IFFM-E flow injection CL analyser (Xi'an Remex Electronic instrument High-Tech Ltd, China) was equipped with an automatic injection system and a detection system. PTFE tubes (0.8 mm i.d.) were used to connect all of the components in the flow system. 50 mg β-CD/Cs–MGO–SMIP and non-imprinted polymer (β-CD/Cs–MGO–SNIP) were added to a capillary, and were collected between a pump and CL analyser with PTFE tubes as recognition elements. A magnet was placed on the side to fix β-CD/Cs-MGO-SMIP (β-CD/Cs–MGO–SNIP) to the capillary to prevent its run off with the solution. When BSA solution was run through the capillary, BSA molecules could be absorbed by β-CD/Cs–MGO–SMIP selectively, a CL signal I1 was obtained, while β-CD/Cs–MGO–SNIP could not absorb BSA molecules, another CL signal I2 was obtained. The difference ΔI = I2 − I1 was the concentration of BSA in a linear relationship. In this way, a specific recognition and measurement system was obtained. XRD measurements were performed on a D8 focus spectrometer (Brooke AXS, Germany). A FEI QUANTA FEG250 field emission scanning electron microscope (SEM, USA) was employed to observe the morphology of the nanoparticles. A vibrating-sample magnetometer (VSM) (MAG-3110, Freescale) was used at 300 K to characterize the magnetic properties of β-CD/Cs–MGO–SMIP.2.3 Preparation of MGO
MGO was prepared by a modified Hummers' method.22 Firstly, 120 mL of H2SO4 was added into 5.0 g of natural graphite powder and 2.5 g of NaNO3. Then, 6.0 g of KMnO4 was added gradually under stirring, and the diluted suspension was stirred at 98 °C. Subsequently, 50 mL of 30% H2O2 was added drop by drop. Finally, the mixture was filtered and washed until the pH = 7.0. MGO was synthesized according to a modified procedure described by our group.23 While suspending 0.5 g of GO in 200 mL of a solution containing 5.1 g of (NH4)2Fe(SO4)2·6H2O and 7.5 g of NH4Fe(SO4)2·12H2O under an N2 atmosphere, the solution was sonicated. Then, 10 mL of 8 mol L−1 NH4OH aqueous solution was added dropwise to precipitate the iron oxides until the pH = 11.5. The reaction was maintained at 80 °C for 30 min. The obtained black precipitate was separated, washed and then dried under vacuum at 60 °C.2.4 Preparation of β-CD/Cs–MGO
In a typical procedure, 0.1 g of newly obtained MGO was added to a molten Cs colloidal acetic acid solution and the pH of the solution was adjusted to be 5.5. Then, 80 mg of β-CD was added to the mixture with vigorous stirring. After that, 3 mL of glutaraldehyde was added. The reaction was carried out at 70° C for 60 min under constant mechanical stirring. The precipitate was isolated under a magnetic field and washed with double-distilled water. The obtained β-CD/Cs–MGO composites were then dried under vacuum.2.5 Preparation of β-CD/Cs–MGO–SMIP
A modified procedure described in our work and in previous literature was used to synthesize β-CD/Cs–MGO–SMIP.23 The preparation process is shown in Fig. 1. MBA (64 mg) was dissolved in 35 mL of PBS solution by ultrasonication. Subsequently, 32 mg of BSA was dissolved in the solution. Then, 15 mL of β-CD/Cs–MGO (150 mg) dispersed in 10 mL of ethanol and 5 mL of PBS solution by ultrasonication was added to the above solution. The mixture was degassed for 10 min and purged with a nitrogen stream for 10 min. Then, the solution was shaken for 0.5 h to preassemble. By adding 30 mg of APS and 0.2 mL of TEMED to the mixture, polymerization was initiated, and continued under shaking at 25 °C for 10 min. The particles were collected by magnetic separation and washed with NaCl solution until no BSA was present in the supernatant. Finally, the particles were washed with PBS solution and dried. β-CD/Cs–MGO–SNIP was prepared in exactly the same way, but without the addition of BSA.2.6 Adsorption properties of β-CD/Cs–MGO–SMIP and β-CD/Cs–MGO–SNIP
Adsorption isotherm: 100 mg of β-CD/Cs–MGO–SMIP and 100 mg of β-CD/Cs–MGO–SNIP nanoparticles were placed into separate 10 mL centrifuge tubes. Then, 8.0 mL of different concentration solutions of BSA was added to the tubes and the tubes were shaken at 25 °C for 1 h. After magnetic separation, the concentration of the supernatant in the tubes was determined by a CL instrument and the adsorption capacities were calculated.Rebinding dynamics: 100 mg of β-CD/Cs–MGO–SMIP and β-CD/Cs–MGO–SNIP nanoparticles were dispersed in 8.0 mL of 2.0 mg mL−1 BSA solution. Immediately, the solution was shaken at 25 °C for 2.5 min, 5 min, 7.5 min, 10 min, 20 min, 40 min and 60 min. After magnetic separation, the concentration of the supernatant in the tube was determined by a CL instrument and the adsorption capacities were calculated. The adsorption capacity was calculated from the following formula:
| Qe = (c0 − ce)V/m |
2.7 Selectivity studies of β-CD/Cs–MGO–SMIP and β-CD/Cs–MGO–SNIP
100 mg of β-CD/Cs–MGO–SMIP and 100 mg of β-CD/Cs–MGO–SNIP nanoparticles were placed into separate 10 mL centrifuge tubes. Then, 8.0 mL of 2.0 mg mL−1 BSA solution, bovine hemoglobin (BHb) solution, lysozyme (Lys) solution, cytochrome C (CyC) solution and ribonuclease A (RNase A) solution were added into the tubes. The solutions were shaken at 25 °C for 30 min. After magnetic separation, the concentration of the supernatant in the tubes was determined and the adsorption capacity of β-CD/Cs–MGO–SMIP and β-CD/Cs–MGO–SNIP nanoparticles towards BSA, BHb, Lys, CyC and RNase A was determined.3 Results and discussion
3.1 Characterization of GO, MGO and β-CD/Cs–MGO
Fig. 2A illustrates the XRD patterns of the obtained GO and MGO particles. Obviously, MGO displayed several diffraction rings at 2θ = 30.2°, 35.6°, 43.4°, 53.3°, 57.5° and 63.1° which corresponded to (220), (311), (400), (422), (511), and (440), the six indices of the Fe3O4 inverse spinel structure, and the characteristic peak of GO at 2θ = 10.1°. The satisfying results provided remarkable support that MGO was successful prepared. As indicated in Fig. 2B, the magnetization measurements demonstrated that the saturation magnetization of β-CD/Cs–MGO–SMIP was 18.9 emu g−1, which was sufficient to meet the needs of the magnetic separation process. Fig. 2C shows the Fourier Transform Infrared (FTIR) Spectroscopy of MGO and β-CD/Cs–MGO. The peaks at 1400–1600 cm−1 are characteristic peaks of the benzene ring. In the spectrum of MGO, the peak at 620 cm−1 is characteristic of Fe3O4 nanoparticles. The absorption band with strong intensity and shape at 1736 cm−1 was attributed to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band. In the spectrum of β-CD/Cs–MGO, the broad and moderate intensity peak at 620 cm−1 is a characteristic peak for Fe3O4 nanoparticles and the flexural vibration of N–H in acid amide. The peak at 1126 cm−1 was attributed to the stretching vibration of C–N. When reacted with –NH2, the peak of C
O band. In the spectrum of β-CD/Cs–MGO, the broad and moderate intensity peak at 620 cm−1 is a characteristic peak for Fe3O4 nanoparticles and the flexural vibration of N–H in acid amide. The peak at 1126 cm−1 was attributed to the stretching vibration of C–N. When reacted with –NH2, the peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1736 cm−1 shifted to 1680 cm−1, which confirmed the reaction of MGO and β-CD/Cs.
O at 1736 cm−1 shifted to 1680 cm−1, which confirmed the reaction of MGO and β-CD/Cs.
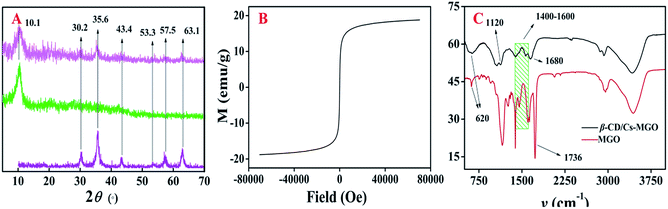 | ||
| Fig. 2 XRD patterns of obtained GO and MGO (A), VSM magnetization curves of β-CD/Cs–MGO–SMIP (B) and FTIR of MGO and β-CD/Cs–MGO (C). | ||
Scanning electron microscopy (SEM) was used to characterize the surface morphology of the GO, MGO and β-CD/Cs–MGO. As shown in Fig. 3A, a wrinkled and thin film is observed in the image of GO. A loose 3D network structure consisting of 2D GO sheets is displayed clearly. Fig. 3B clearly reveals that Fe3O4 nanoparticles were decorated on the GO surface which did not alter the microstructure of GO significantly, and the incorporation of Fe3O4 on the GO sheets enabled the maximum utilization of GO. Obviously, a large amount of Fe3O4 nanoparticles were immobilized onto the GO films. As shown in Fig. 3C, the obvious difference on the surface of β-CD/Cs–MGO compared with MGO indicated interaction between GO and β-CD, Cs. A higher surface area which served as a supporting interface to obtain more binding sites in SMIP was obtained after the immobilization of β-CD and Cs onto the MGO.
3.2 Batch binding properties of β-CD/Cs–MGO–SMIP
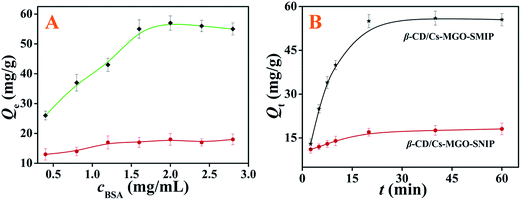
The adsorption results are shown in Fig. 4. The adsorption isotherm (Fig. 4A) for BSA increased with the increase of the BSA concentration, before reaching a maximum of 58 mg g−1 (Qm = 58 mg g−1). Obviously, β-CD/Cs–MGO–SMIP exhibited a significant imprinting effect, binding more than three times as much BSA compared to the SNIP which was prepared under the same conditions. As we could observe in Fig. 4B, both the SMIP and SNIP particles could reach their maximum adsorption within 20 min for the imprinting cavities on the surface or in the proximity of the surface of β-CD/Cs–MGO–SMIP.where ce (mg mL−1) is the equilibrium concentration of BSA, Qe (mg g−1) is the adsorption capacity, Qtm (mg g−1) is the theoretical saturation adsorption capacity, kL is the Langmuir constant, kF is the binding energy constant and n is the Freundlich constant.
 | ||
| Fig. 5 Adsorption isotherm equation of β-CD/Cs–MGO–SMIP for BSA: Langmuir model (A) and Freundlich model (B). | ||
As shown in Fig. 5, the fit of the Langmuir model provided a substantially higher correlation coefficient. Accordingly, Langmuir isotherm equations (R2 = 0.9879) were more appropriate than the Freundlich isotherm equations (R2 = 0.9302) at the presented temperature. Therefore, the adsorption of BSA on β-CD/Cs–MGO–SMIP was monolayer uniform adsorption and the imprinting sites on the surface of β-CD/Cs–MGO–SMIP had a homogeneous distribution. The theoretical adsorption capacity (Qtm) of β-CD/Cs–MGO–SMIP was obtained as 60 mg g−1, which was similar to the experimental result, 58 mg g−1.
3.3 Batch binding properties of β-CD/Cs–MGO–SMIP
Fig. 7A shows the adsorption capacities of β-CD/Cs–MGO–SMIP and β-CD/Cs–MGO–SMIP nanoparticles towards BSA, BHb, Lys, CyC and RNase A in PBS solutions with a feed concentration of 0.4 mg mL−1. Evidently, all SMIPs exhibited greater binding capacity compared to their NIPs due to their macro-imprinting cavities which would absorb biomacromolecules, while no imprinting cavities existed in the SNIPs. Certainly from the figure, BSA-β-CD/Cs–MGO–SMIP bound much more BSA compared to the other four proteins, which was of significant interest in terms of their selectivity.3.4 Analytical performance of β-CD/Cs–MGO–SMIP–CL biosensor
BSA could enhance the CL intensity of luminol in the presence of H2O2 and NaOH. In order to establish the optimum experimental conditions, the parameters affecting the CL performance were studied. Firstly, the effect of NaOH on the CL intensity was investigated and the optimal concentration of NaOH was fixed at 4.0 × 10−5 mol L−1. Subsequently, the effect of H2O2 on the CL intensity was studied and the approached maximum intensity value was obtained 1.2 × 10−2 mol L−1. Then, the concentration of luminol employed was 5.0 × 10−3 mol L−1 in subsequent experiments. Thus, under the above conditions, the calibration curve of CL intensity against BSA concentration was found to be linear in the range of 5.0 × 10−7–1.0 × 10−4 mg mL−1, and the correlation coefficient was 0.9877 with a detection limit of 1.1 × 10−7 mg mL−1, as shown in Fig. 7B. As shown in Table 1, the result showed that our work was superior in both linear range and detection limit compared with other methods.3.5 Selectivity studies of the β-CD/Cs–MGO–SMIP–CL biosensor
In order to study the recognition properties of the biosensor using β-CD/Cs–MGO–SMIP nanoparticles towards BSA, the CL intensity of solutions containing high amounts of other substances such as Mg2+, L-tryptophan, Lys and BHb was researched. It was evident from Fig. 7C that 370 times Mg2+ (compared to the concentration of BSA) would interfere in the CL biosensor, while an interference of 700 times Mg2+ was observed in the β-CD/Cs–MGO–SMIP–CL biosensor. As anticipated, L-tryptophan exhibited a higher interference compared with Mg2+. While 80 times Lys interfering the detection of BSA apparently in simple CL, interference could be eliminated (200 times) when employing β-CD/Cs–MGO–SMIP selectivity adsorbing BSA. Certainly, the interference of BHb was relatively more significant. Evidently, the CL method exhibited a significant interference effect of more than triple the concentration ratio compared to the β-CD/Cs–MGO–SMIP–CL biosensor. The high selectivity of the biosensor was accounted for the specific structure, namely imprinting cavies, which were located on the surface of SMIP matrix and could recognize and separate biomacromolecule BSA.3.6 Application of β-CD/Cs–MGO–SMIP–CL biosensor
Table 2 illustrates the application of the β-CD/Cs–MGO–SMIP–CL biosensor in samples. The results showed that the β-CD/Cs–MGO–SMIP–CL biosensor was capable of detecting BSA with good recoveries ranging from 94% to 106%. As indicated, the proposed β-CD/Cs–MGO–SMIP–CL biosensor was highly accurate, precise and selective, and it could be used for the analysis of samples. The application of the proposed biosensor for measuring samples demonstrated the feasibility of β-CD/Cs–MGO–SMIP in a CL biosensor.| Sample | c (10−6 mg mL−1) | Added (10−6 mg mL−1) | Found (n = 6) (10−6 mg mL−1) | RSD% | Recovery (%) |
|---|---|---|---|---|---|
| 1# | 1.9 | 5.0 | 7.1 | 3.5 | 104 |
| 2# | 5.4 | 5.0 | 10.7 | 4.0 | 106 |
| 3# | 7.7 | 5.0 | 12.4 | 3.3 | 94 |
4 Conclusion
In this paper, using a β-CD/Cs–MGO nanocomplex as a new supporting material in the preparation process of SMIPs, a new CL biosensor for BSA based on β-CD/Cs–MGO–SMIP has been proposed. The maximum adsorption capacity of β-CD/Cs–MGO–SMIP was 58 mg g−1 and the obtained β-CD/Cs–MGO–SMIP followed the Langmuir isotherm equation and pseudo-second order sorption kinetics when binding BSA. Fast mass transfer, a promoted rate of removal of the biomolecule, and excellent recognition and adsorption ability for the imprinting cavities situated at the surface or in proximity to the surface of the β-CD/Cs–MGO was achieved, which enabled easy access to the target protein molecules. The combination of the high selectivity of SMIP based on the strong recognition effect between SMIP and BSA and the highly sensitive CL determination method made the proposed biosensor perform excellently in the determination of BSA. Based on this, future work will focus on the preparation of synthetic receptor materials as SMIP elements with higher adsorption capacity and selectivity for the fabrication of biomimetic CL biosensors.References
- D. R. Persaud and A. B. Mendoza, Bovine serum albumin and insulin-dependent diabetes mellitus: is cow's milk still a possible toxicological causative agent of diabetes?, Food Chem. Toxicol., 2004, 42, 707–714 CrossRef CAS PubMed.
- M. B. Gholivand, A. R. Jalalvand, H. C. Goicoechea, R. Gargallo and T. Skov, Chemometrics: an important tool for monitoring interactions of vitamin B7 with bovine serum albumin with the aim of developing an efficient biosensing system for the analysis of protein, Talanta, 2015, 132, 354–365 CrossRef CAS PubMed.
- H. E. Indyk, B. D. Gill and D. C. Woollard, An optical biosensor-based immunoassay for the determination of bovine serum albumin in milk and milk products, Int. Dairy J., 2015, 47, 72–78 CrossRef CAS PubMed.
- I. S. Turan, O. Yilmaz, B. Karatas and E. U. Akkaya, A sensitive and selective chemiluminogenic probe for palladium, RSC Adv., 2015, 5, 34535–34540 RSC.
- R. Panahi, E. V. Farahani and S. A. Shojaosadati, Separation of l-lysine from dilute aqueous solution using molecular imprinting technique, Biochem. Eng. J., 2007, 35, 352–356 CrossRef CAS PubMed.
- Y. Liu, B. Cao, P. Jia, C. Luo, J. An, J. Chang and K. Pan, Layer-by-layer surface molecular imprinting on polyacrylonitrile nanofiber mats, J. Phys. Chem. A, 2015, 119, 6661–6667 Search PubMed.
- K. Kim, K. Kim, J. H. Ryu and H. Lee, Chitosan-catechol: a polymer with long-lasting mucoadhesive properties, Biomaterials, 2015, 52, 161–170 CrossRef CAS PubMed.
- M. Monier, D. M. Ayad, Y. Wei and A. A. Sarhan, Preparation of cross-linked chitosan/glyoxal molecularly imprinted resin for efficient chiral resolution of aspartic acid isomers, Biochem. Eng. J., 2010, 51, 140–146 CrossRef CAS PubMed.
- S. S. Voznesenskiy, A. A. Sergeev, A. Y. Mironenko, S. Y. Bratskaya and Y. N. Kulchin, Integrated-optical sensors based on chitosan waveguide films for relative humidity measurements, Sens. Actuators, B, 2013, 188, 482–487 CrossRef CAS PubMed.
- S. S. Alias, Z. M. Ariff and A. A. Mohamad, Porous membrane based on chitosan-SiO2 for coin cell proton battery, Ceram. Int., 2015, 41, 5484–5491 CrossRef CAS PubMed.
- Y. Yang, Y. Long, Q. Cao, K. Li and F. Liu, Molecularly imprinted polymer using β-cyclodextrin as functional monomer for the efficient recognition of bilirubin, Anal. Chim. Acta, 2008, 606, 92–97 CrossRef CAS PubMed.
- M. L. Bender and M. Komiyama, Cyclodextrin Chemistry, Springer, Berlin, 1978 Search PubMed.
- S. M. Ng and R. Narayanaswamy, Molecularly imprinted β-cyclodextrin polymer as potential optical receptor for the detection of organic compound, Sens. Actuators, B, 2009, 139, 156–165 CrossRef CAS PubMed.
- K. Kano, R. Nishiyabu, T. Asada and Y. Kuroda, Static and dynamic behavior of 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complexes of cyclodextrins and charged porphyrins in aqueous organic media, J. Am. Chem. Soc., 2002, 124, 9937–9944 CrossRef CAS PubMed.
1 inclusion complexes of cyclodextrins and charged porphyrins in aqueous organic media, J. Am. Chem. Soc., 2002, 124, 9937–9944 CrossRef CAS PubMed. - S. Wang, B. Wang, H. Si, J. Shan and X. Yang, Preparation of magnetic molecularly imprinted polymer beads and their recognition for baicalein, RSC Adv., 2015, 5, 8028–8036 RSC.
- J. Wang, G. Zhao, L. Jing, X. Peng and Y. Li, Facile self-assembly of magnetite nanoparticles on three-dimensional graphene oxide-chitosan composite for lipase immobilization, Biochem. Eng. J., 2015, 98, 75–83 CrossRef CAS PubMed.
- P. Gupta and R. N. Goyal, Graphene and co-polymer composite based molecularly imprinted sensor for ultratrace determination of melatonin in human biological fluids, RSC Adv., 2015, 5, 40444–40454 RSC.
- B. M. García, A. Polovitsyn and M. P. Iwan Moreels, Efficient charge transfer in solution-processed PbS quantum dot-reduced graphene oxide hybrid materials, J. Mater. Chem. C, 2015, 3, 7088–7095 RSC.
- H. Vovusha, S. Sanyal and B. Sanyal, Interaction of nucleobases and aromatic amino acids with graphene oxide and graphene flakes, J. Phys. Chem. Lett., 2013, 4, 3710–3718 CrossRef CAS.
- W. Chen, P. Yi, Y. Zhang, L. Zhang, Z. Deng and Z. Zhang, Composites of aminodextran-coated Fe3O4 nanoparticles and graphene oxide for cellular magnetic resonance imaging, ACS Appl. Mater. Interfaces, 2011, 3, 4085–4091 CAS.
- T. Jiang, L. Yan, L. Zhang, Y. Li, Q. Zhao and H. Yin, Fabrication of a novel graphene oxide/β-FeOOH composite and its adsorption behavior for copper ions from aqueous solution, Dalton Trans., 2015, 44, 10448–10456 RSC.
- W. S. Hummers and R. E. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- H. Y. He, G. Q. Fu, Y. Wang, Z. H. Chai, Y. Z. Jiang and Z. L. Chen, Imprinting of protein over silica nanoparticles via surface graft copolymerization using low monomer concentration, Biosens. Bioelectron., 2010, 26, 760–765 CrossRef CAS PubMed.
- X. Zhu, Y. Hu and A. Gong, Investigation on the interaction of tetrachloride fluorescein-bovine serum albumin-β-cyclodextrin and the determination of protein by flow injection analysis, Anal. Chim. Acta, 2007, 592, 24–29 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |