Thermoresponsive hydrophobic copolymer brushes modified porous monolithic silica for high-resolution bioseparation†
Kenichi Nagase*a,
Jun Kobayashia,
Akihiko Kikuchib,
Yoshikatsu Akiyamaa,
Hideko Kanazawac and
Teruo Okano*a
aInstitute of Advanced Biomedical Engineering and Science, Tokyo Women's Medical University, TWIns, 8-1 Kawadacho, Shinjuku, Tokyo 162-8666, Japan. E-mail: nagase.kenichi@twmu.ac.jp; tokano@twmu.ac.jp; Fax: +81-3-3359-6046; Tel: +81-3-5367-9945 ext. 6201, 6224
bDepartment of Materials Science and Technology, Tokyo University of Science, 6-3-1 Niijuku, Katsushika, Tokyo 125-8585, Japan
cFaculty of Pharmacy, Keio University, 1-5-30 Shibakoen, Minato, Tokyo 105-8512, Japan
First published on 23rd July 2015
Abstract
Thermoresponsive hydrophobic copolymer, poly(N-isopropylacrylamide-co-n-butyl methacrylate) [P(IPAAm-co-BMA)], brushes were prepared on porous monolithic silica rods through surface-initiated atom transfer radical polymerization (ATRP). The monolithic silica surface was modified with the ATRP-initiator [(chloromethyl)phenylethyl]trimethoxysilane (CPTMS) via silanization performed by flowing a CPTMS toluene solution into the monolithic silica rod column. ATRP was performed by flowing a reaction solution containing IPAAm and BMA monomers, and ATRP catalyst CuCl/CuCl2/tris[2-(N,N-dimethylamino)ethyl]amine in 2-propanol into the monolithic silica. Characterization by CHN elemental analysis, X-ray photoelectron spectroscopy (XPS), and gel permeation chromatography revealed that dense P(IPAAm-co-BMA) brushes were formed on the monolith surface. Chromatographic analysis using benzoic acids and insulin peptides indicated that the brush-modified monolithic silica was able to separate these analytes with high resolution and in short analysis times, owing to the effective interaction between the modified polymer brushes and the analyte. These results indicated that the present P(IPAAm-co-BMA) brush modified monolithic silica is an effective separation tool for analyzing bio-related molecules.
Introduction
The thermoresponsive polymer poly(N-isopropylacrylamide) (PIPAAm) is widely used in various types of biomedical applications1–8 owing to its temperature-modulated hydrophilic and hydrophobic alteration near body temperature, with a low critical transition temperature of 32 °C.9 Accordingly, PIPAAm has been used in temperature triggered drug delivery systems and thermally modulated biocatalyst systems. Also, substrates modified with this polymer exhibit a temperature dependent wettability change. This property has been used to create cell culture substrates that allow temperature modulated cell adhesion and detachment, leading to the fabrication of “cell sheets”.10,11 Various types of cell sheets have already been used in clinical trials. Moreover, thermoresponsive chromatography systems using PIPAAm and its copolymers have been shown to be a particularly promising application of PIPAAm modified surfaces.12,13 In these systems, a PIPAAm or copolymer modified chromatographic stationary phase, such as silica beads14 or polymer beads,15 is used as the stationary phase. Because the surface hydrophobicity can be modulated by an external temperature change, the hydrophobic interaction between the stationary surface and the analyte can be modulated. Unlike conventional reversed phase chromatography systems, organic solvents are not required to modulate the elution profile of the analytes. Thus, these systems have the advantages of preserving the biological activity of the analytes and reduced environmental load. Various polymer modification methods have been investigated to improve the separation efficiency of this thermoresponsive chromatography system, such as coupling reactions,14 radical polymerization,16 and surface-initiated atom transfer radical polymerization (ATRP).17,18 ATRP has been found to be the most effective polymer modification method, because the polymerization allows for accurate control of the polymerization and formation of densely packed polymer brush structures on substrates.19–33 A densely packed polymer brush structure leads to a larger amount of polymer grafting on surface, resulting in strong interaction between the copolymer and analytes.17,18The copolymerization of functional monomers into PIPAAm is an effective approach for improving the separation efficiency of the system. Several functional copolymer brushes have been investigated by introducing different types of co-monomers for various bioseparation purposes.34 By incorporating ionic monomers into PIPAAm brushes, acidic or basic biomolecules can be separated through electrostatic interaction modulated by temperature changes. In a similar manner, incorporation of hydrophobic monomers, such as n-butyl methacrylate (BMA), also allows relatively hydrophilic analytes to be separated through strong hydrophobic interactions between BMA-incorporating thermoresponsive brushes and analytes. However, in several cases, excessive hydrophobic interaction between the hydrophobic thermoresponsive copolymer brushes and the analytes results in a longer separation time.
In analytical science, monolithic silica rod columns have been paid much attention as an alternative for the conventional packed-bed columns.35–40 Monolithic silica has an interconnected structure, which allows for low backpressure of the flowing mobile phase owing to a high permeability. Also, the structure of monolithic silica rod contributes to a more effective use of surface area than in the case of silica beads, leading to enhanced surface–analyte interaction. Thus, the linear velocity of the mobile phase among the silica rods can be increased, allowing high-speed separation with high resolution. Actually, monolithic silica modified with PIPAAm41 or ionic thermoresponsive copolymers42,43 can perform high resolution separation of steroids, adenosine nucleotides, catecholamine, or angiotensin through hydrophobic interaction or electrostatic interaction. Thus, if monolithic silica could be used as the base material, the issue of longer separation time could be solved using hydrophobic thermoresponsive copolymer brushes.
In the present paper, the surfaces of monolithic silica rods were modified with P(IPAAm-co-BMA) through surface-initiated ATRP. Characterization of the prepared monolithic silica was performed by X-ray photoelectron spectroscopy (XPS), CHN elemental analysis, gel permeation chromatography (GPC), scanning electron microscopy (SEM), and nitrogen adsorption measurement. The separation efficiency of prepared columns was evaluated by the elution of benzoic acid and insulin fragments.
Experimental
Materials
IPAAm was kindly provided from Kohjin (Tokyo, Japan) and purified by recrystallization from n-hexane. BMA, obtained from Wako Pure Chemicals (Osaka, Japan), was purified by distillation. CuCl, CuCl2, tris(2-aminoethyl)amine (TREN), toluene, 2-propanol, NaOH, hydrochloric acid, formaldehyde, formic acid, different benzoic acids, and α-chloro p-xylene were also obtained from Wako Pure Chemicals. Tris[2-(N,N-dimethylamino)ethyl]amine (Me6TREN) was synthesized from TREN, according to a previous report.44 [(Chloromethyl)phenylethyl]trimethoxysilane (mixed meta and para isomers) was obtained from Gelest (Morrisville, PA, USA). Monolithic silica rod columns (MonoBis 50 mm × 3.2 mm i.d., surface area: 71.8 m2 g−1; apparent mesopore size; 30 nm) were obtained from Kyoto Monotech (Kyoto, Japan). Silica beads with a diameter of 5 μm, a specific surface area of 100 m2 g−1, and a pore size of 300 Å were obtained from Chemco Scientific (Osaka, Japan).Initiator modification of silica surfaces
The surfaces of the monolithic silica were modified with ATRP-initiator through a silane coupling reaction (Fig. 1(A)). Before the reaction, the monolithic silica rod was placed into a humidified container (relative humidity of approximately 75%) for 18 h, because humidification of a silica surface is required for effective silanization.45–47 The ATRP-initiator solution was prepared by dissolving 6 mL of [(chloromethyl)phenylethyl]trimethoxysilane (CPTMS) into 14 mL of toluene in a glass vessel. The prepared ATRP initiator solution was circulated into the monolithic silica rod column at a flow rate of 0.1 mL min−1 for 16 h using a HPLC pump (PU-980, JASCO, Tokyo). After the silane coupling reaction, the monolithic silica rod column was rinsed with toluene and acetone, and dried in a high vacuum oven at 110 °C for 2 h. ATRP-initiator immobilized silica beads were also prepared according to previous reports.18,24 Briefly, 31.4 g of silica beads was placed into a round bottom flask, the relative humidity of which was set at 60% for 4 h. Then, CPTMS (8.251 mL) was dissolved in 612.8 mL of toluene, and the prepared solution was reacted with the silica beads under continuous stirring for 16 h. After the reaction, the silica beads were rinsed with toluene, methanol, dichloromethane, and acetone, and dried at 110 °C for 2 h.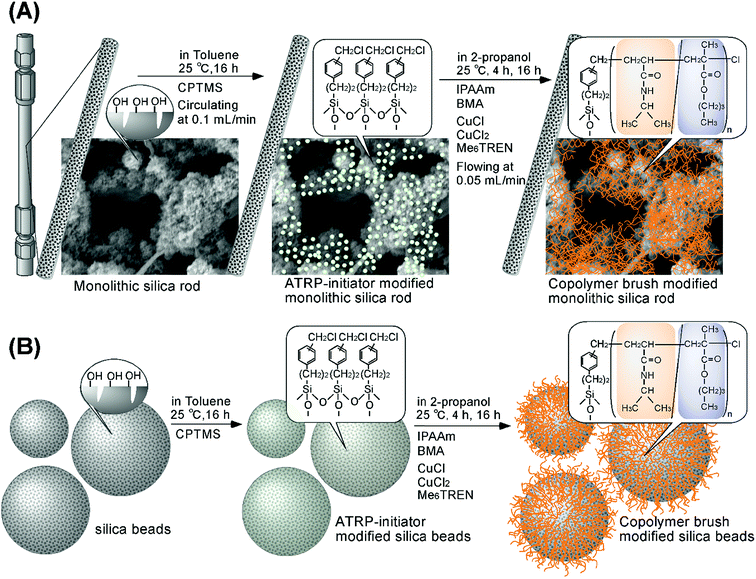 | ||
| Fig. 1 Scheme for preparation of P(IPAAm-co-BMA)-brush-grafted monolithic silica rod (A) and silica beads (B) using surface-initiated ATRP. | ||
Copolymer brush grafting by ATRP
A thermoresponsive copolymer possessing hydrophobic moieties, P(IPAAm-co-BMA), was grafted onto the monolithic silica rod through surface-initiated ATRP (Fig. 1(A)). First, IPAAm (13.9 g, 122 mmol, 95 mol%) and BMA (0.917 g, 6.45 mmol, 5 mol%) were dissolved in 2-propanol (85.6 mL). The dissolved oxygen in the monomer solution was removed by argon gas bubbling for 1 h. CuCl (0.168 g, 1.70 mmol) and CuCl2 (23.0 mg, 0.171 mmol) were added to the monomer solution under an argon atmosphere and stirring for 10 min, after which Me6TREN (0.44 g, 1.91 mmol) was added to the solution and stirred for 10 min to form a CuCl/CuCl2/Me6TREN ATRP catalyst system. The initiator-modified column and the reaction solution were placed into a glove box in which the oxygen concentration was below 0.1%. The ATRP reaction solution was flowed into the initiator-modified monolithic silica column at a flow rate of 0.05 mL min−1 using a HPLC pump (PU-980). The ATRP reaction time was set to 4 or 16 h, which are the copolymer grafting conditions used to obtain short and long copolymer brushes, respectively.18 After the ATRP, the monolithic silica rod was rinsed with methanol, acetone, and water, and dried at 50 °C for 3 h.For comparison, silica beads modified with the same copolymer brushes were also prepared through surface-initiated ATRP (Fig. 1(B)). IPAAm (4.62 g, 40.8 mmol, 95 mol%) and BMA (306 mg, 2.15 mmol, 5 mol%) were dissolved in 42.8 mL of 2-propanol, and the solution was deoxygenated under bubbling argon gas for 1 h. CuCl (84.7 mg, 0.856 mmol) and CuCl2 (11.5 mg, 0.0855 mmol) were added to the monomer solution under argon atmosphere and 10 min stirring, after which Me6TREN (0.22 g, 0.955 mmol) was added to the solution and stirred for 10 min to form the CuCl/CuCl2/Me6TREN ATRP catalyst system. Both the initiator-modified silica beads (1.0 g) in glass vessel and the reaction solution were put into a glove bag. The oxygen concentration in the glove bag was reduced to 0.1% by repeated vacuuming and argon gas introduction. The reaction solution was poured into the glass vessel and sealed. The ATRP was allowed to proceed for 4 and 16 h under continuous shaking using a mechanical shaker (SB-400, AS ONE, Tokyo, Japan). After the ATRP, the beads were rinsed with acetone, methanol, EDTA solution, and water, and dried in a vacuum oven at 50 °C for 3 h.
Characterization of initiator- and copolymer modified silica
The amount of initiator and copolymer on the modified silica matrices was measured using a CHN elemental analyzer (PE2400 II, PerkinElmer, Waltham, MA, USA). The density of initiator on the silica surface was calculated as follows:17
 | (1) |
 | (2) |
The molecular weight of copolymer grafted on the silica matrix was measured by retrieving the grafted copolymer through treatment with concentrated sodium hydroxide solution (10 mol L−1) overnight by dissolving silica surface.48 The obtained solution was neutralized with hydrochloric acid, and undissolved silica was removed by filtration using a filter membrane (Omnipore, Millipore, Billerica, Massachusetts, USA). The filtrate was dialyzed against pure water using a dialysis membrane (molecular weight cut-off (MWCO): 1000; Rancho Dominguez, CA, USA) for 7 days with daily water changes. The purified copolymer was obtained by freeze drying of the solution. The number averaged molecular weight (Mn) and dispersity (Đ) estimated by dividing the weight averaged molecular weight (Mw) by the number averaged molecular weight (Mn) of the copolymers were measured using a size exclusion chromatography system (GPC-8020 II, Columns: TSKgel SuperAW2500, TSKgel SuperAW3000, and TSKgel SuperAW4000, Tosoh, Tokyo, Japan). DMF containing 50 mM LiCl was used as the mobile phase, with a flow rate of 1.0 mL min−1. Calibration was performed using poly(ethylene glycol) standards. Elution profiles were monitored using a refractometer.
The graft density of the copolymer on the silica matrix was estimated using
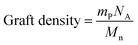 | (3) |
The surface elemental composition was determined using XPS (K-Alpha, Thermo Fisher Scientific, Waltham, MA, USA). Excitation X-rays were produced using a monochromatic A1 Kα1,2 source with a take-off angle of 90°. The surface morphologies of the initiator and copolymer modified silica matrices were observed by SEM (S-4300, Hitachi, Tokyo, Japan).
Surface area and pore size distributions were determined using a nitrogen-adsorption measurement apparatus (Belsorp 18 Plus-HT, BEL Japan, Osaka, Japan), to investigate the effect of polymer grafting on the surface area and pore size distributions of the samples.
Wettability change of thermoresponsive copolymer brushes
Because the measurement of the wettability of silica beads and monolithic silica is difficult owing to their structure, glass beads modified with same copolymer brushes were prepared for measuring the thermoresponsive wettability change.49 Glass beads having a diameter of 212–250 μm were washed with hydrochloric acid at 90 °C for 3 h. Then, the surface of the glass beads was modified with CPTMS using the same silane coupling reaction as that use to couple the initiator to the silica beads. Copolymer brushes were grafted onto the glass beads (20.0 g) by surface-initiated ATRP using the same conditions as those for the silica beads. The amount of grafted copolymer on the surface of the glass beads was determined by micro nitrogen analysis using a nitrogen microanalyzer (ND-100, Mitsubishi Chemical Analytech, Tokyo, Japan). The amount of grafted copolymer on the glass beads was calculated by the following equation:50
 | (4) |
Milli-Q water (5 μL) was dropped onto a glass plate (24.0 mm × 50 mm; Matsunami Glass, Osaka, Japan). A small amount of the glass beads were put on the droplet, a microscope cover glass slide (24 mm × 24 mm; Matsunami Glass) was pressed over the mixture, and then the glass beads were squeezed towards the liquid–air boundary. The contact angle was measured from photographs obtained using a phase-contrast microscope (ECLIPS TE2000-U, Nikon, Tokyo, Japan) and a digital camera (FinePix, S2Pro, Nikon). The temperature of the glass beads was regulated with a thermoplate (Thermo Plate, Tokai Hit, Fujinomiya, Japan). Data are expressed as the mean of five measurements with standard deviation.
Synthesis of hydrophobized thermoresponsive copolymer by ATRP
Solution-phase ATRP was used to prepare P(IPAAm-co-BMA) for characterization. The copolymerization was performed using the same ATRP procedure as that of the silica beads except that α-chloro-p-xylene (53.4 mg, 380 μmol) was used in place of the silica beads, and the BMA composition was varied from 0 mol% to 10 mol%. After the ATRP, the reaction solution was dialyzed against an EDTA solution for 2 days and Milli-Q water for 5 days with daily water changes. The purified copolymer was obtained by freeze drying.The molecular weight and dispersity of the copolymer were determined using the gel permeation chromatography. The composition of the prepared copolymers was determined by 1H NMR (UNITYINOVA 400 MHz spectrometer, Varian, Palo Alto, CA, USA), using DMF-d7 as the solvent. The phase transition profile of the copolymer solution (10 mg mL−1) was observed using a UV-visible spectrophotometer (V-660, JASCO, Tokyo, Japan). The LCST of the copolymer was defined as the temperature at which the transmittance was 90%.
Temperature modulated elution of analytes
The copolymer modified silica beads were packed into a stainless steel column (50 mm × 4.6 mm). A suspension of silica beads in a mixture of water and methanol (50 vol%/50 vol%) was introduced into a column packer (Tosho) connected to a stainless steel column. Then, a mixed solvent of water and ethanol was passed through the column at 350 kg cm−2 for 1 h, after which the column was rinsed with flowing Milli-Q water overnight.Each column was connected to an HPLC system (PU-980, UV-970, AS-950-10, Borwin analysis software, JASCO). Sodium benzoate, methyl benzoate, ethyl p-aminobenzoate, ethyl benzoate, methyl p-hydroxybenzoate, and phenol were used as small molecular analytes. These compounds are used as preservatives or fragrances in food or cosmetics. Their properties are summarized in Table S1.†51 Each sample was prepared by dissolving 3 mg of the compound into ethanol (10 mL). The elution behavior of benzoic acids and phenol in the columns at various temperatures was monitored at 254 nm with a 1 mL min−1 flow of Milli-Q water as the mobile phase. The column temperature was controlled by immersing the column into a thermostated water bath controlled with constant-temperature water circulator (CTA400, Yamato, Tokyo, Japan).
Insulin fragments, insulin chain A, insulin chain B, and insulin, were used as large molecular analytes. Their properties are shown in Table S2.† Insulin chain A (1.0 mg) and insulin chain B (1.0 mg) were dissolved in 3.0 mL of phosphate buffer (PB; pH 7.0, 66.7 mM). Insulin (1.0 mg) was dissolved in 2.0 mL of 1.0 M HCl. The elution behavior of the insulin fragments was monitored at 215 nm with a 1.0 mL min−1 flow of 66.7 mM phosphate buffer (pH 7.0). The column temperature was controlled using the same thermostated water bath.
Results and discussion
Characterization of thermoresponsive copolymer
P(IPAAm-co-BMA), a thermoresponsive polymer possessing hydrophobic moieties, was prepared by solution-phase ATRP. Characterization of the copolymer samples was performed by 1H NMR, GPC, and by observing its phase transition behavior. The results are summarized in Table 1 and Fig. 2. All copolymer samples were labelled using the abbreviated monomer names, reaction time, and BMA feed composition. “IP” and “B” correspond to IPAAm and BMA, respectively. “4” and “-5” in 4IPB-5 represent the ATRP reaction time (h) and the BMA feed molar ratio (%) in ATRP, respectively. 1H NMR analysis revealed that the composition of BMA in the samples was slightly higher than the feed composition. This indicated that BMA has higher reactivity that IPAAm under the ATRP conditions used. Our previous reports also indicated that a higher composition of methacrylate monomer was obtained compared with acrylamide monomer under similar ATRP conditions.52–54 The dispersity of the prepared copolymers was relatively small, indicating that the ATRP was well-controlled. However, a slight increase in Đ was observed when the feed BMA composition was increased. The ATRP catalyst used in the present study was CuCl/CuCl2/Me6TREN, which is typically used for the polymerization of acrylamide derivatives, not methacrylate derivatives.55,56 Additionally, several reports have indicated that bromine-initiator is more suitable for initiation of methacrylate monomer than chloride-initiator with a chloride based catalyst.57,58 Thus, the control of the polymerization was slightly reduced with the incorporation of BMA. Regarding the ATRP reaction time, 16IPB-5 had a larger molecular weight compared with 4IPB-5, indicating that ATRP continued between 4 and 16 h.| Codea | IPAAm/BMA (molar ratio) | Mnc | Dispersity Mw/Mnc | LCSTd | ||
|---|---|---|---|---|---|---|
| In feed | In copolymerb | In water | In 66.7 mmol L−1 PB | |||
| a All samples were prepared through using solution phase ATRP and named using abbreviated monomer names. “IP” and “B” represent IPAAm and BMA, respectively.b According to 1H-NMR results.c Measured by GPC using a 50 mmol L−1 LiCl solution in DMF as the mobile phase.d Defined as the temperature where the sample solution had a transmittance of 90%. | ||||||
| IPB-0 | 100/0 | 100/0 | 7100 | 1.11 | 32.1 | |
| IPB-1 | 99.0/1.0 | 99.5/0.54 | 7200 | 1.12 | 23.9 | |
| IPB-3 | 97.0/3.0 | 97.2/2.80 | 6900 | 1.14 | 15.5 | |
| IPB-5 | 95.0/5.0 | 94.7/5.26 | 7300 | 1.18 | 11.4 | |
| IPB-7 | 93.0/7.0 | 91.5/8.52 | 6400 | 1.22 | 8.9 | |
| IPB-10 | 90.0/10.0 | 88.9/11.1 | 6100 | 1.27 | — | |
| 4IPB-5 | 95.0/5.0 | 93.9/6.06 | 4600 | 1.17 | 9.31 | 9.50 |
| 16IPB-5 | 95.0/5.0 | 93.9/6.10 | 6200 | 1.19 | 10.5 | 11.4 |
 | ||
| Fig. 2 Phase-transition profiles of P(IPAAm-co-BMA) prepared using various BMA feed molar ratios in Milli-Q water (A), and P(IPAAm-co-BMA)s prepared using different ATRP reaction time (B). | ||
To determine the optimum BMA composition, the phase transition behavior of copolymers with various BMA compositions was investigated. All copolymers except IPB-10 were dissolved in Milli-Q water under cooling with an ice bath. However, IPB-10 was unable to be dissolved even in an ice bath. This was caused by the excessive hydrophobic properties of BMA in the copolymer. As shown in Fig. 2(A), the phase transition temperature of the copolymer decreased with increasing BMA composition, because the incorporated BMA enhanced the dehydration of the copolymer. Although IPB-7 exhibited the lowest phase transition temperature (8.9 °C), we considered IPB-5 to be the most suitable copolymer for a thermoresponsive chromatographic stationary phase, because a column temperature under 10 °C is not practical for HPLC system operation.
Comparing the phase transition behavior of 4IPB-5 and 16IPB-5 (Fig. 2(B)), 16IPB-5, the longer copolymer, exhibited a slightly higher phase transition temperature than 4IPB-5, the shorter copolymer. Also, the phase transition curve obtained using PB was shifted to lower temperature that obtained using Milli-Q water, because of the salting out effect induced by the PB species.
Characterization of copolymer-modified silica
The surface elemental composition of the silica matrices was measured by XPS. The results are summarized in Table 2. Samples were named according to the structure of the base material, copolymer composition, and ATRP reaction time. IM and IB represent initiator-modified “monolith” and “beads”, respectively. “IP” and “B” represent “IPAAm” and “BMA”, respectively. Also “4” and “16” indicate ATRP reaction time (h) and “-5” indicate the BMA feed molar composition during ATRP. The chemical bonds of the base material surfaces were investigated by deconvolution of the C1s carbon peaks (Fig. S1†) according to previous reports. Additional peaks attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the copolymer were observed in the spectra of the copolymer-modified silica matrices (IM-4IPB-5, IM-16IPB-5, IB-4IPB-5, and IB-16IPB-5), but not in the spectra of the initiator modified-matrices (IM and IB), indicating that the copolymer was successfully grafted onto the initiator-modified surface though surface-initiated ATRP. Additionally, the carbon and nitrogen content increased and silicon content decreased after ATRP, confirming the success of the surface initiated-ATRP, because the increased carbon and nitrogen originated from the IPAAm and BMA monomer. Furthermore, the surfaces with longer copolymer brushes (IM-16IPB-5, IB-16IPB-5) exhibited relatively larger carbon and nitrogen content and smaller silicon content compared with those of the surfaces with shorter copolymer brushes (IM-4IPB-5, IB-4IPB-5). This is because the short copolymer brushes were very thin and thus allowed the base silica material to be detected.
O bond of the copolymer were observed in the spectra of the copolymer-modified silica matrices (IM-4IPB-5, IM-16IPB-5, IB-4IPB-5, and IB-16IPB-5), but not in the spectra of the initiator modified-matrices (IM and IB), indicating that the copolymer was successfully grafted onto the initiator-modified surface though surface-initiated ATRP. Additionally, the carbon and nitrogen content increased and silicon content decreased after ATRP, confirming the success of the surface initiated-ATRP, because the increased carbon and nitrogen originated from the IPAAm and BMA monomer. Furthermore, the surfaces with longer copolymer brushes (IM-16IPB-5, IB-16IPB-5) exhibited relatively larger carbon and nitrogen content and smaller silicon content compared with those of the surfaces with shorter copolymer brushes (IM-4IPB-5, IB-4IPB-5). This is because the short copolymer brushes were very thin and thus allowed the base silica material to be detected.
| Codea | Atom (%)d | N/C ratio | ||||
|---|---|---|---|---|---|---|
| C | N | O | Si | Cl | ||
| a All samples were named according to the silica structure and grafted copolymer component. IM and IB represent “initiator-modified monolithic silica-rod” and “initiator-modified silica beads”, respectively. “4” and “16” indicated ATRP reaction time (h) used to preparing each sample. “IP” and “B” represent IPAAm and BMA, respectively, and “-5” represents BMA feed composition in ATRP.b Estimated atomic composition of IPAAm.c Estimated atomic composition of BMA.d Data from three separate experiments are shown as mean ± SD. | ||||||
| IM | 10.9 ± 1.12 | 0.39 ± 0.40 | 54.3 ± 0.92 | 33.0 ± 0.15 | 1.41 ± 0.34 | — |
| IM-4IPB-5 | 49.4 ± 2.40 | 6.93 ± 0.50 | 31.1 ± 1.70 | 11.2 ± 4.63 | 1.36 ± 0.29 | 0.140 |
| IM-16IPB-5 | 58.9 ± 4.94 | 8.15 ± 2.08 | 22.5 ± 1.89 | 8.96 ± 0.89 | 1.54 ± 0.61 | 0.138 |
| IB | 24.5 ± 7.83 | 0.35 ± 0.25 | 47.3 ± 2.74 | 26.4 ± 5.33 | 1.48 ± 0.19 | — |
| IB-4IPB-5 | 62.0 ± 0.95 | 8.07 ± 0.46 | 20.7 ± 1.06 | 8.23 ± 0.50 | 1.00 ± 0.12 | 0.130 |
| IB-16IPB-5 | 66.9 ± 0.56 | 8.86 ± 0.46 | 17.8 ± 0.28 | 5.82 ± 0.44 | 0.65 ± 0.44 | 0.132 |
| Calcd of IPAAmb | 75.0 | 12.5 | 12.5 | — | — | 0.167 |
| Calcd of BMAc | 80.0 | — | 20.0 | — | — | 0 |
The amounts of initiator and copolymer on the monolithic silica matrices were determined by CHN elemental analysis, and their molecular weight and graft density was estimated by GPC measurements (Table 3). Slightly larger amounts of initiator were present on the monolithic silica surfaces than the silica bead surfaces, although a higher concentration of initiator solution was reacted with the monolithic silica rod. This is probably attributed to the difference in surface silica reactivity between monolithic silica rod and silica beads, which affected the silane coupling reaction. Larger amounts of copolymer were grafted onto the monolithic silica rod and silica beads than on similar copolymer-modified silica beads prepared by conventional radical polymerization.59 This is because a dense packing of copolymer brushes was achieved on the silica surfaces with surface-initiated ATRP. A larger difference in the molecular weights of copolymer brushes prepared with long and short reaction times was observed for the monolithic silica compared with that of the silica beads. This was attributed to the different ATRP procedures used for the monolithic silica rod and silica beads. For the modification of the monolithic silica rod, the ATRP reaction solution was flowed over the monolithic silica rod, supplying fresh reaction solution containing unreacted monomer and catalyst to the surface. In contrast, the silica beads were reacted with the reaction solution inside a glass vessel. As the ATRP reaction proceeded, the monomer was consumed and a certain amount of catalyst was deactivated in the batch reaction. Thus, the increase in the molecular weight of copolymer on the surface of the beads with proceeding reaction time was smaller than that on the monolithic silica.
| Codea | Elemental composition (%)b | Immobilized initiatorc (μmol m−2) | Grafted copolymerc (mg m−2) | Mnd | Dispersity Mw/Mnd | Graft density (chains per nm2) | ||
|---|---|---|---|---|---|---|---|---|
| C | H | N | ||||||
| a All samples were named according to the silica structure and grafted copolymer component. IM and IB represent initiator-modified “monolith silica rods” and “silica beads”, respectively. “4” and “16” indicate ATRP reaction time (h) used to prepare each sample. “IP” and “B” represent IPAAm and BMA, respectively, and “-5” represents BMA feed composition in ATRP.b Determined by elemental analysis (n = 3).c Estimated from the carbon composition.d Determined by GPC using a 50 mmol L−1 LiCl solution in DMF as the mobile phase. | ||||||||
| IM | 2.97 ± 0.07 | 0.06 ± 0.01 | 0.44 ± 0.07 | 3.94 | ||||
| IM-4IPB-5 | 13.8 ± 0.53 | 1.22 ± 0.23 | 2.34 ± 0.08 | 3.05 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.54 | 0.090 | |
| IM-16IPB-5 | 20.9 ± 0.14 | 2.31 ± 0.22 | 3.40 ± 0.01 | 5.87 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.33 | 0.116 | |
| IB | 4.80 ± 0.03 | 0.18 ± 0.15 | 0.22 ± 0.04 | 4.66 | ||||
| IB-4IPB-5 | 18.1 ± 0.04 | 1.86 ± 0.10 | 2.53 ± 0.04 | 2.95 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.33 | 0.112 | |
| IB-16IPB-5 | 19.0 ± 0.03 | 2.18 ± 0.03 | 2.66 ± 0.02 | 3.22 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.33 | 0.121 | |
The copolymers cleaved from their silica matrices exhibited larger molecular weight and dispersity Đ than those of corresponding copolymers prepared by solution phase ATRP, as displayed in Table 1 (see Fig. S2† for GPC chart of cleaved copolymer). In solution phase ATRP, almost all the added initiator was able to initiate, while a certain amount of immobilized initiator was unable to initiate owing to steric hindrance induced by previously immobilized monomer or the geometry of the silica. Also, the larger dispersity Đ is probably attributed to the porous geometry of the silica matrices. Monomers and catalyst diffusion inside pore is partially restricted compared with outer surface of pore, leading to the difference in molecular weight between outside and inside pores. Another possible reason for the relatively larger dispersity is the hydrolysis of the IPAAm side chains by the concentrated NaOH solution. Thus, to determine the amount of IPAAm side chains remaining after the NaOH treatment, the 1H NMR spectrum of the retrieved copolymers was observed (shown in Fig. S3 and Table S3†). A peak at 3.9 ppm attributed to the IPAAm methine proton was observed, indicating that IPAAm side chain remained after immersion in the NaOH solution.
A high graft density, approximately 0.10 chain per nm2, was observed, indicating that grafted copolymer form densely packed polymer brush structure on silica surfaces.
The surface morphologies of non-modified and copolymer modified silica matrices were observed using SEM (Fig. 3 and S4†). These SEM images indicated that the monolithic silica retained its macro-porous structure after the copolymer grafting, despite the larger amount of copolymer grafted on the monolithic silica matrices. This result indicated that an adequate flowpath of the mobile phase was maintained in the monolithic silica rod. To further investigate the flow conditions of the mobile phase, Milli-Q water was flowed into prepared columns at various temperatures and the backpressure of the HPLC pump was monitored (Fig. 4). The backpressure of the column decreased with increasing temperature, because the hydrated and extended copolymer brushes became dehydrated and shrunken. The back pressure of the non-modified monolithic silica also decreased with increasing temperature, although the decrease in pressure was small compared with that observed with the copolymer modified monolithic silica and beads. This is attributed to the decrease in the viscosity of the water mobile phase with increasing temperature. The backpressure of the monolithic silica rod column remained low compared with that of the silica beads packed column, although an increase in back pressure was observed after copolymer grafting. These results indicated that copolymer modification using surface-initiated ATRP was achieved without clogging the macropores of the monolithic silica, allowing the copolymer modified monolithic silica columns to be used as low backpressure HPLC columns.
 | ||
Fig. 3 (A-1, A-2) SEM images of long P(IPAAm-co-BMA)-brush-grafted monolithic silica rod (IM-16IPB-5 in Table 2); (B-1, B-2) SEM images of short P(IPAAm-co-BMA)-brush-grafted monolithic silica rod (IM-4IPB-5); (C-1, C-2) SEM images of non-modified monolithic silica rod; (D-1, D-2) SEM images of long P(IPAAm-co-BMA)-brush-grafted silica beads (IB-16IPB-5); (E-1, E-2) SEM images of short P(IPAAm-co-BMA)-brush-grafted silica beads (IB-4IPB-5); (F-1, F-2) SEM images of non-modified silica beads. Background patterns in (D-1, D-2), (E-1, E-2), and (F-1, F-2) are double-sided tape used to affix the samples. Images numbered (A-2), (B-2), (C-2), (D-2), (E-2), and (F-2) are high-resolution (×15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 magnification). 000 magnification). | ||
Also, adsorption–desorption isotherms were obtained to determine the actual surface area before and after copolymer grafting60–63 (Fig. 5). BET plots60 and pore size distributions determined using the Barrett–Joyner–Halenda method61 are shown in Fig. S5 and S6,† respectively. A summary of the data is shown in Table 4. Surface area and peak diameter decreased after the grafting of copolymer on these silica substrates. These results are attributed to infilling of the pores of the silica surfaces by copolymer. However, the obtained peak diameter and total pore volume of the monolithic silica did not change after copolymer grafting, probably because the monolithic silica rod contained mesopores and macro-pores, leading to imprecise measurement of the pore size.
 | ||
| Fig. 5 Nitrogen adsorption–desorption isotherms of the prepared monolithic silica rod (A) and silica beads (B). The silica matrices abbreviations are given in Table 2, and the obtained surface area is given in Table 3. | ||
| Codea | Surface areab (m2 g−1) | Total pore volumec (cm3 g−1) | Peak pore diameterc (nm) | Averaged peak diameter (nm) |
|---|---|---|---|---|
| a All samples were named according to the silica structure and grafted copolymer component. IM and IB represent initiator-modified “monolith silica rods” and “silica beads”, respectively. “4” and “16” indicate ATRP reaction time (h) used to prepare each sample. “IP” and “B” represent IPAAm and BMA, respectively, and “-5” represents BMA feed composition in ATRP.b Calculated using the Brunauer–Emmett–Teller (BET) method.c Calculated using the Barrett–Joyner–Halenda (BJH) method. | ||||
| IM-4IPB-5 | 55 | 0.23 | 51.1 | 16.1 |
| IM-16IPB-5 | 55 | 0.14 | 51.1 | 9.2 |
| Non-modified monolithic silica-rod | 77 | 0.20 | 51.1 | 11.6 |
| IB-4IPB-5 | 47 | 0.11 | 13.9 | 7.9 |
| IB-16IPB-5 | 43 | 0.10 | 12.1 | 7.7 |
| Non-modified silica beads | 92 | 0.38 | 32.6 | 16.2 |
Thermoresponsive wettability change of copolymer brushes
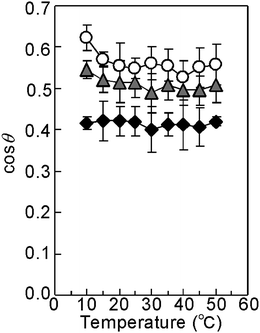
To measure the temperature dependent wettability change of the copolymer brushes, P(IPAAm-co-BMA) brushes were prepared on the surface of glass beads using the same protocol as that used for the silica beads, and the contact angle was measured (Fig. 6 and S7†). The amount of grafted copolymer on the glass beads was found to be 4.22 mg m−2 and 5.20 mg m−2 after reaction times of 4 h and 16 h, respectively, measured by nitrogen microanalysis (data shown in Table S4†).50 The copolymer brush-modified glass beads exhibited larger cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, determined by contact angle measurements as shown in Fig. 6, than the initiator modified glass beads, indicating that the copolymer brushes were relatively hydrophilic compared with the ATRP initiator. The copolymer brushes also exhibited relatively higher hydrophobicity than the PIPAAm homopolymer brushes described in a previous report,50 attributed to the incorporation of hydrophobic BMA into the polymer brushes. The contact angle (cos
θ, determined by contact angle measurements as shown in Fig. 6, than the initiator modified glass beads, indicating that the copolymer brushes were relatively hydrophilic compared with the ATRP initiator. The copolymer brushes also exhibited relatively higher hydrophobicity than the PIPAAm homopolymer brushes described in a previous report,50 attributed to the incorporation of hydrophobic BMA into the polymer brushes. The contact angle (cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) of long copolymer brush-modified glass beads was higher than that of the shorter copolymer brush-modified beads, indicating that the longer copolymer brushes were hydrophilic than the short copolymer brushes. The cos
θ) of long copolymer brush-modified glass beads was higher than that of the shorter copolymer brush-modified beads, indicating that the longer copolymer brushes were hydrophilic than the short copolymer brushes. The cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ of the copolymer brushes decreased with increasing temperature as shown in Fig. 6, attributed to the dehydration of the grafted copolymer brush. A larger decrease was observed around the LCST of the copolymer.
θ of the copolymer brushes decreased with increasing temperature as shown in Fig. 6, attributed to the dehydration of the grafted copolymer brush. A larger decrease was observed around the LCST of the copolymer.
Elution profiles of analytes from copolymer brush
The temperature-dependent elution profiles of the prepared copolymer brush-modified monolithic silica rod and silica beads columns were observed using small molecular analytes, benzoic acids and phenol (Fig. 7). The dependence of retention time on temperature is shown in Fig. 8. The resolution between methyl benzoate and ethyl benzoate are shown in Fig. S8.† All analytes were retained by the hydrophobic copolymer brushes, and the separation resolution increased with column temperature. These results indicated that the copolymer brushes interacted with the analytes through hydrophobic interaction, and that the interaction increased with the dehydration of the copolymer induced by the elevating temperature. Additionally, these results also demonstrate that the prepared copolymer brush modified column could exhibit modulated analyte elution profiles simply through a temperature change and without the addition of organic solvents to the mobile phase, unlike conventional reversed phase chromatography matrices. | ||
| Fig. 7 Elution profiles of benzoic acids and phenol using poly(IPAAm-co-BMA)-brush-grafted monolithic silica and silica beads as packing materials. (A) Long and (B) short P(IPAAm-co-BMA)-brush-grafted monolithic silica rod columns (IM-16IPB-5 and IM-4IPB-5 in Table 2), and (C) long and (D) short P(IPAAm-co-BMA)-brush-grafted silica bead columns (IB-16IPB-5 and IB-4IPB-5 in Table 2). The mobile phase was Milli-Q water. Flow rate was 1.0 mL min−1. Peak (1) represents sodium benzoate; (2), phenol; (3), methyl benzoate; (4), ethyl p-aminobenzoate; (5), ethyl benzoate; (6), methyl p-hydroxybenzoate. Because of its high-speed separation abilities, the timescale for the IM-IPB column is expanded compared with those of the IB-IPB columns. | ||
 | ||
| Fig. 8 Temperature dependences of benzoic acids and phenol retention times on (A) long and (B) short P(IPAAm-co-BMA)-brush-grafted monolithic silica rod columns (IM-16IPB-5 and IM-4IPB-5 in Table 2), and (C) long and (D) short P(IPAAm-co-BMA)-brush-grafted silica bead columns (IB-16IPB-5 and IB-4IPB-5 in Table 2). Closed circles, sodium benzoate; open triangles, phenol; closed diamonds, methyl benzoate; closed squares, ethyl p-aminobenzoate; closed triangles, ethyl benzoate; open circles, methyl p-hydroxybenzoate. | ||
Smaller molecule analytes diffused into the extended copolymer brushes at low temperature, leading to uneven elution and wider peaks, while at high temperature, the copolymer brushes dehydrated and shrank, preventing the diffusion of the analytes into the copolymer brush layers.17 Wider peaks were observed for the long copolymer brush-modified monolithic silica and silica beads (IM-16IPB-5 and IB-16IPB-5) compared with those of the short copolymer brushes (IM-4IPB-5 and IB-4IPB-5), because low molecular weight analytes tend to diffuse more into a longer copolymer brush than a short copolymer brush layer. As for the separation efficiency, the monolithic silica columns exhibited short retention time with high separation efficiency, although similar copolymer brushes were modified on the different types of silica surface. The high efficiency of the monolithic silica was also indicated by the higher resolution of the columns (Fig. S8†). This is attributed to the structural difference in the channel size for the flowing mobile phase between the monolithic silica and silica bead columns. The monolithic silica rod had a three-dimensionally interconnected structure, which reduced the flow resistance and diffusion path length of the analytes. Thus, the mobile phase flowed with relatively high linear velocity in the monolithic silica rod, than in the beads-packed column, and uneven analyte elution was suppressed. SEM images (Fig. 3) also suggested that the structure of the monolithic silica rod contributed to a more effective use of the surface area than in the case of the silica beads, leading to enhanced surface–analyte interaction. Thus, the present copolymer brush-modified monolithic silica rod column allowed for high-speed separation with high resolution. Also, the resolution increased with temperature, because the retention time of the analytes increased and the peak width decreased with increasing temperature, induced by dehydration of the grafted copolymer. Furthermore, the effect of long-period use was investigated with a continuous flowing mobile phase at 30 °C and repeated injection of benzoic acids (Fig. S9 and Table S5†). The benzoic acid resolution and the back pressure scarcely changed, indicating that the prepared column had high stability.
To investigate the separation efficiency of peptides on the prepared columns, insulin fragments, insulin chain A, insulin chain B, and insulin, were used as analytes. The obtained elution profiles are shown in Fig. 9 and the changes in retention time are shown in Fig. 10. Chromatograms obtained at 10 and 50 °C are shown in Fig. S10.† The retention time of the insulin peptides increased with temperature, and the mixture of these insulin peptides were separated. The hydrophobic copolymer brushes on the silica base materials dehydrated and become hydrophobic with increasing temperature, leading to hydrophobic interaction between copolymer brush and peptides. Also, the peak width of the eluted peptides was relatively small compared with that of the benzoic acids, because larger molecular analytes did not tend to diffuse into the copolymer brushes, leading to even elution and narrower peaks. The monolithic silica columns (IM-16IPB-5 and IM-4IPB-5) performed baseline separation of insulin fragments while silica beads packed columns (IB-16IPB-5 and IB-4IPB-5) did not. This result indicated that the monolithic rod columns had a higher separation efficiency for peptides than the bead-packed columns, owing to the properties of the monolithic silica rod structure. Also, IM-4IPB-5 exhibited higher separation efficiency than IM-16IPB-5. This is attributed to the strong hydrophobic properties of the short copolymer brushes compared with that of the long copolymer brushes (Fig. 6), which led to a stronger hydrophobic interaction with the peptides.
 | ||
| Fig. 9 Elution profiles of insulin fragments using poly(IPAAm-co-BMA)-brush-grafted monolithic silica and silica beads as packing materials. (A) Long and (B) short P(IPAAm-co-BMA)-brush-grafted monolithic silica rod columns (IM-16IPB-5 and IM-4IPB-5 in Table 2), and (C) long and (D) short P(IPAAm-co-BMA)-brush-grafted silica beads columns (IB-16IPB-5 and IB-4IPB-5 in Table 2). The mobile phase was 66.7 mM phosphate buffer (pH 7.0). Flow rate was 1.0 mL min−1. Peak no. 1, 2, and 3 represent insulin chain A, insulin chain B, and insulin, respectively. | ||
 | ||
| Fig. 10 Temperature dependences of insulin fragment retention times on (A) long and (B) short P(IPAAm-co-BMA)-brush-grafted monolithic silica rod columns (IM-16IPB-5 and IM-4IPB-5 in Table 2, respectively), and (C) long and (D) short P(IPAAm-co-BMA)-brush-grafted silica beads columns (IB-16IPB-5 and IB-4IPB-5 in Table 2). Open circles, closed diamonds, and closed triangles represent insulin, insulin chain A, and insulin chain B, respectively. | ||
The above results indicated that monolithic silica rods modified with long copolymer brushes can separate small molecular analytes with a high resolution in short analysis time. Additionally, for separating relatively larger molecules, such as peptides, short copolymer brush-modified monolithic silica rods are suitable for high-resolution separation. Therefore, monolithic silica rod columns grafted with P(IPAAm-co-BMA) brush can separate both smaller molecular analytes and peptides using copolymer brushes of suitable length.
Conclusion
Densely-packed hydrophobized thermoresponsive copolymer, P(IPAAm-co-BMA), brushes were successfully grafted onto monolithic silica surfaces through surface-initiated ATRP, performed using a flowing monomer and catalyst solution. The molecular weight of the modified copolymer brushes was modulated by changing the ATRP reaction time, i.e., duration of monomer solution flowing into the monolithic silica rod. Contact angle measurements revealed that short copolymer brushes were more hydrophobic than long copolymer brushes. The elution profiles of benzoic acids and phenol through the columns indicated that monolithic silica rod grafted with P(IPAAm-co-BMA) brush separated these analytes with high resolution and shorter analysis time compared with packed columns containing silica beads modified with the same copolymer. This result is attributed to the decreased diffusion path length and high linear velocity of the mobile phase in the monolithic silica rod. Additionally, copolymer-modified monolithic silica rod columns separated insulin peptides with a higher resolution than that of corresponding silica bead-packed columns. Therefore, the present monolithic silica rod column grafted with P(IPAAm-co-BMA) brushes is an effective bioseparation tool for analyzing both small molecular analytes and peptides with high resolution and short analysis time, though strong thermally modulated hydrophobic interaction.Acknowledgements
This research was partly financially supported by the Development of New Environmental Technology Using Nanotechnology Project of the National Institute of Environmental Science (NIES), commissioned from the Ministry of Environment, Japan, and subsidies from the Kumagai Foundation for Science and Technology, Grants-in-Aid for Scientific Research (C) No. 26420714 from the Japan Society for the Promotion of Science (JSPS), Japan.References
- E. S. Gil and S. M. Hudson, Prog. Polym. Sci., 2004, 29, 1173–1222 CrossRef CAS PubMed.
- A. S. Hoffman and P. S. Stayton, Prog. Polym. Sci., 2007, 32, 922–932 CrossRef CAS PubMed.
- M. Ulbricht, Polymer, 2006, 47, 2217–2262 CrossRef CAS PubMed.
- J. F. Mano, Adv. Eng. Mater., 2008, 10, 515–527 CrossRef CAS PubMed.
- T. Mori and M. Maeda, Langmuir, 2003, 20, 313–319 CrossRef.
- Y.-J. Kim, M. Ebara and T. Aoyagi, Angew. Chem., Int. Ed., 2012, 51, 10537–10541 CrossRef CAS PubMed.
- Y. Gao, X. Li and M. J. Serpe, RSC Adv., 2015, 5, 44074–44087 RSC.
- V. A. Ganesh, A. Baji and S. Ramakrishna, RSC Adv., 2014, 4, 53352–53364 RSC.
- M. Heskins and J. E. Guillet, J. Macromol. Sci., Chem., 1968, 2, 1441–1455 CrossRef CAS PubMed.
- M. Yamato, Y. Akiyama, J. Kobayashi, J. Yang, A. Kikuchi and T. Okano, Prog. Polym. Sci., 2007, 32, 1123–1133 CrossRef CAS PubMed.
- Y. Haraguchi, T. Shimizu, M. Yamato and T. Okano, RSC Adv., 2012, 2, 2184–2190 RSC.
- A. Kikuchi and T. Okano, Macromol. Symp., 2004, 207, 217–228 CrossRef CAS PubMed.
- H. Kanazawa and T. Okano, J. Chromatogr. A, 2011, 1218, 8738–8747 CrossRef CAS PubMed.
- H. Kanazawa, K. Yamamoto, Y. Matsushima, N. Takai, A. Kikuchi, Y. Sakurai and T. Okano, Anal. Chem., 1996, 68, 100–105 CrossRef CAS PubMed.
- A. Mizutani, K. Nagase, A. Kikuchi, H. Kanazawa, Y. Akiyama, J. Kobayashi, M. Annaka and T. Okano, J. Chromatogr. A, 2010, 1217, 522–529 CrossRef CAS PubMed.
- T. Yakushiji, K. Sakai, A. Kikuchi, T. Aoyagi, Y. Sakurai and T. Okano, Anal. Chem., 1999, 71, 1125–1130 CrossRef CAS.
- K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa and T. Okano, Langmuir, 2007, 23, 9409–9415 CrossRef CAS PubMed.
- K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa and T. Okano, Langmuir, 2008, 24, 511–517 CrossRef CAS PubMed.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef CAS PubMed.
- T. Wu, K. Efimenko and J. Genzer, J. Am. Chem. Soc., 2002, 124, 9394–9395 CrossRef CAS PubMed.
- H. Tu, C. E. Heitzman and P. V. Braun, Langmuir, 2004, 20, 8313–8320 CrossRef CAS PubMed.
- S. Balamurugan, S. Mendez, S. S. Balamurugan, M. J. O'Brien II and G. P. López, Langmuir, 2003, 19, 2545–2549 CrossRef CAS.
- S. Edmondson, V. L. Osborne and W. T. S. Huck, Chem. Soc. Rev., 2004, 33, 14–22 RSC.
- D. Xiao and M. J. Wirth, Macromolecules, 2002, 35, 2919–2925 CrossRef CAS.
- R. Iwata, P. Suk-In, V. P. Hoven, A. Takahara, K. Akiyoshi and Y. Iwasaki, Biomacromolecules, 2004, 5, 2308–2314 CrossRef CAS PubMed.
- Y. Xia, X. Yin, N. A. D. Burke and H. D. H. Stover, Macromolecules, 2005, 38, 5937–5943 CrossRef CAS.
- P. Hemström, M. Szumski and K. Irgum, Anal. Chem., 2006, 78, 7098–7103 CrossRef PubMed.
- S. Peng and B. Bhushan, RSC Adv., 2012, 2, 8557–8578 RSC.
- J. Jin, J. Liu, X. Lian, P. Sun and H. Zhao, RSC Adv., 2013, 3, 7023–7029 RSC.
- X. Wang, R. Berger, J. I. Ramos, T. Wang, K. Koynov, G. Liu, H.-J. Butt and S. Wu, RSC Adv., 2014, 4, 45059–45064 RSC.
- X. Huang and M. J. Wirth, Anal. Chem., 1997, 69, 4577–4580 CrossRef CAS.
- X. Huang, L. J. Doneski and M. J. Wirth, Anal. Chem., 1998, 70, 4023–4029 CrossRef CAS PubMed.
- N. Ayres, Polym. Rev., 2011, 51, 138–162 CrossRef CAS PubMed.
- K. Nagase, J. Kobayashi and T. Okano, J. R. Soc., Interface, 2009, 6, S293–S309 CrossRef CAS PubMed.
- H. Minakuchi, K. Nakanishi, N. Soga, N. Ishizuka and N. Tanaka, Anal. Chem., 1996, 68, 3498–3501 CrossRef CAS PubMed.
- N. Tanaka, H. Kobayashi, K. Nakanishi, H. Minakuchi and N. Ishizuka, Anal. Chem., 2001, 73, 420A–429A CrossRef CAS.
- N. Tanaka, H. Nagayama, H. Kobayashi, T. Ikegami, K. Hosoya, N. Ishizuka, H. Minakuchi, K. Nakanishi, K. Cabrera and D. Lubda, J. High Resolut. Chromatogr., 2000, 23, 111–116 CrossRef CAS.
- G. Guiochon, J. Chromatogr. A, 2007, 1168, 101–168 CrossRef CAS PubMed.
- O. Nunez, K. Nakanishi and N. Tanaka, J. Chromatogr. A, 2008, 1191, 231–252 CrossRef CAS PubMed.
- S. Miyazaki, M. Takahashi, M. Ohira, H. Terashima, K. Morisato, K. Nakanishi, T. Ikegami, K. Miyabe and N. Tanaka, J. Chromatogr. A, 2011, 1218, 1988–1994 CrossRef CAS PubMed.
- K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa and T. Okano, Langmuir, 2011, 27, 10830–10839 CrossRef CAS PubMed.
- K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa and T. Okano, Biomacromolecules, 2014, 15, 1204–1215 CrossRef CAS PubMed.
- K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa and T. Okano, ACS Appl. Mater. Interfaces, 2013, 5, 1442–1452 CAS.
- M. Ciampolini and N. Nardi, Inorg. Chem., 1966, 5, 41–44 CrossRef CAS.
- S. R. Wasserman, Y. T. Tao and G. M. Whitesides, Langmuir, 1989, 5, 1074–1087 CrossRef CAS.
- S. Onclin, B. J. Ravoo and D. N. Reinhoudt, Angew. Chem., Int. Ed., 2005, 44, 6282–6304 CrossRef CAS PubMed.
- M. J. Wirth and H. O. Fatunmbi, Anal. Chem., 1992, 64, 2783–2786 CrossRef CAS.
- K. Nagase, A. Mizutani Akimoto, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa and T. Okano, J. Chromatogr. A, 2011, 1218, 8617–8628 CrossRef CAS PubMed.
- Z. Hórvölgyi, S. Németh and J. H. Fendler, Langmuir, 1996, 12, 997–1004 CrossRef.
- K. Nagase, N. Mukae, A. Kikuchi and T. Okano, Macromol. Biosci., 2012, 12, 333–340 CrossRef CAS PubMed.
- C. Hansch, L. Albert and D. Hoekman, in Exploring QSAR: Hydrophobic, Electronic and Steric Constant, American Chemical Society, 1995 Search PubMed.
- K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa and T. Okano, Biomacromolecules, 2008, 9, 1340–1347 CrossRef CAS PubMed.
- K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, M. Annaka and T. Okano, Biomacromolecules, 2010, 11, 215–223 CrossRef CAS PubMed.
- K. Nagase, M. Kumazaki, H. Kanazawa, J. Kobayashi, A. Kikuchi, Y. Akiyama, M. Annaka and T. Okano, ACS Appl. Mater. Interfaces, 2010, 2, 1247–1253 CAS.
- S. G. Gaynor, J. Qiu and K. Matyjaszewski, Macromolecules, 1998, 31, 5951–5954 CrossRef CAS.
- G. Masci, L. Giacomelli and V. Crescenzi, Macromol. Rapid Commun., 2004, 25, 559–564 CrossRef CAS PubMed.
- K. Matyjaszewski, J.-L. Wang, T. Grimaud and D. A. Shipp, Macromolecules, 1998, 31, 1527–1534 CrossRef.
- K. Matyjaszewski, D. A. Shipp, J.-L. Wang, T. Grimaud and T. E. Patten, Macromolecules, 1998, 31, 6836–6840 CrossRef CAS.
- K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa and T. Okano, ACS Appl. Mater. Interfaces, 2012, 4, 1998–2008 CAS.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309–319 CrossRef CAS.
- E. P. Barrett, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373–380 CrossRef CAS.
- Y. Wang, B. Du, X. Dou, J. Liu, B. Shi, D. Wang and H. Tang, Colloids Surf., A, 2007, 307, 16–27 CrossRef CAS PubMed.
- T. Sreethawong, S. Chavadej, S. Ngamsinlapasathian and S. Yoshikawa, Colloids Surf., A, 2007, 296, 222–229 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11038f |
| This journal is © The Royal Society of Chemistry 2015 |