Optimized solid phase extraction methodology for separation, and spectrophotometric determination of boron using amberlite XAD-16 resin modified with 2-(2-benzothiazolylazo)-4-methoxyphenol
Alaa S. Amin*
Chemistry Department, Faculty of Science, Benha University, Benha, Egypt. E-mail: asamin2005@hotmail.com; Fax: +20 132222578; Tel: +20 552350996
First published on 28th July 2015
Abstract
A highly selective and sensitive spectrophotometric method has been developed to determine trace amounts of boron in various biological and water samples. The method is based on adsorption of B(III) after complexation with 2-(2-benzothiazolylazo)-4-methoxy-phenol (BTAMP) in HOAc–NH4OAc buffer at pH 5.5 on an amberlite XAD-16 resin in the presence of Triton X-114. The retained analyte on the resin can be recovered with 4.0 mL of 2.0 M sulfuric acid and boron is determined spectrophotometrically at λmax 622 nm. Beer’s law is obeyed in the concentration range of 0.05–125 ng mL−1 of B(III) in the measured solution. For more accurate results, the Ringbom optimum concentration range was found to be 0.2–110 ng mL−1. The linear regression equation obtained was A = 0.413C (μg mL−1) + 0.003 (r = 0.9994). The molar absorptivity was calculated to be 4.46 × 105 L mol−1 cm−1 at 622 nm, whereas the Sandell sensitivity was found to be 2.42 ng cm−2. Various parameters such as the effect of pH, reagent concentration, surfactant, and flow rate were studied. The interference of a number of metal ions on the determination of boron has been studied in detail. The proposed method was successfully applied to determine boron concentrations in biological materials, water and ceramic samples. In addition, excellent agreement was observed between the proposed and the reference methods.
Introduction
The essentiality of boron to animals and human beings has not been identified, but it is essential for plants. Boron deficiency affects plant growth and yield, but substantial amounts of boron are toxic to plants and reduce plant yield. Boron has been classified as hazardous by the Agency for Toxic Substances and Disease Registry (ATSDR), and the minimal risk level for boron was given as 0.01 mg per kg per day for oral exposure.1 Therefore, it is suggested that excess boron is toxic for all living organisms,1–3 and, therefore, its determination is important in water, soil, food and some industrial fields, such as metallurgy, electronics, glass manufacture and the nuclear industry.Boron is an element that is difficult to determine. Sah and Brown have reviewed analytical methods for the determination of boron.3 Various spectrometric methods, including atomic absorption spectrometry,3,4 atomic emission spectrometry,3,5 inductively coupled plasma-atomic emission spectrometry,3,6,7 inductively coupled plasma-mass spectrometry,8–12 and X-ray fluorescence spectrometry13 have been reported for the determination of boron in various samples. Advantages and disadvantages of these methods have been well-discussed by Sah and Brown.3 Inductively coupled plasma atomic emission spectrometry (ICP-AES) created a new dimension in boron determination because of its simplicity, sensitivity and multi-element capability. However, ICP-AES suffers from interferences and is not adequately sensitive for some nutritional and medical applications involving animal tissues that are low in boron. Inductively coupled plasma mass spectrometry (ICP-MS) not only overcomes most of the drawbacks of earlier methods, but is also capable of measuring boron isotopes. However, it is time consuming and expensive, and is difficult to use in the routine determination of boron.
In general, the most common methods for the determination of boron concentration are spectrophotometric methods based on various organic colour reagents. However, reagents for the spectrophotometric determination of boron are not ideal14–25 (see Table 1). Most of the reagents require a concentrated H2SO4 medium, so their application for routine boron determination is not satisfactory. The ideal colour reagent would be one that reacts with boron sensitively and selectively in dilute acidic medium.
| Reagent | Reaction conditions | Remarks | Ref. |
|---|---|---|---|
| Ethyl violet | pH = 3.1–4.9 | 1: Benzene extraction | 14 |
| 2: Ag, Au, Re, Sb and Ti interfere | |||
| Crystal violet | pH = 1–2 (H2SO4) | 1: Fe, Hg, W, V and Bi interfere seriously | 14 |
| Carminic acid | 93% H2SO4 | 1: Concentrated medium | 16 |
| 2: Fe, Al, Cu, etc., metal ions interfere | |||
| 1-Hydroxyl-4-p-methylanilino anthraquinone | Concentrated H2SO4 | 1: Concentrated medium | 14 |
| 2: F−, Fe, SO32−, Mn, Cr and V interfere | |||
| Quinalizarin | Concentrated H2SO4 | 1: Concentrated medium | 17 |
| 2: Ge, SO42−, Se and Sb interfere | |||
| Arsenazo I | Concentrated H2SO4 | 1: Concentrated medium | 14 |
| 2: Light-sensitive | |||
| 3: Mg, Fe and Ca interfere seriously | |||
| Arsenazo III | pH = 10 | 1: Light-sensitive | 14 |
| 2: Metal ions interfere seriously | |||
| Curcumin | Concentrated H2SO4 | 1: Concentrated medium | 18 |
| 2: Dichloroethylene extraction | |||
| D-Sorbitol | 1: F−, Mo, Ge and H3PO4 interfere | 19 | |
| 2: Light-sensitive | |||
| Azomethine-H | pH = 6.4–7.0 (HOAc–NH4OAc) | 1: Narrow range of pH | 20 |
| 2: Reagent concentration influences sensitivity seriously | |||
| 3: Fe, Al, Cu, Ti and Zr interfere | |||
| Azomethine-HR | pH = 7.0 (HOAc–NH4OAc) | 1: Narrow range of pH | 21 |
| 2: Reagent concentration influences sensitivity seriously | |||
| 3: Fe, Al, Cu, Ti and Zr interfere |
Amberlite XAD adsorption resins have good physical properties such as porosity, uniform pore size distribution, and high surface area as chemically homogeneous non-ionic structures for large amounts of uncharged compounds, and they have been used as solid sorbents for the enrichment/separation of metal.26–29 In this work a solid phase extraction followed by spectrophotometric determination of boron is described. The method is simple, sensitive, and selective and it allows for the determination of B(III) as low as 0.1 ng mL−1 in water samples.
Experimental
Apparatus
A Perkin-Elmer Lambda 12 UV-vis spectrophotometer (Waltham, MA, USA) with a 10 mm quartz cell was used for all spectral measurements. A funnel tipped glass tube (60 mm × 6 mm) was used as a column for preconcentration. The laboratory glassware (Superior, Germany) and column was kept overnight in a 5.0% nitric acid solution. A Perkin Elmer model 5300 DV ICP-AES (Waltham, MA, USA) was used for all ICP-AES measurements. An Orion research model 601 A/digital ionalyzer pH meter (Tokyo, Japan) was used for checking the pH of solutions.Reagents
All reagents used were of analytical grade and all solutions were prepared with distilled water. All solutions were prepared in polypropylene volumetric flasks. Stock boron solution (1000 μg mL−1) was prepared by dissolving 0.5636 g boric acid (Merck, Darmstadt, Germany) in water and diluting to 100 mL in a volumetric flask. A 2.0 M sulfuric acid solution was prepared by dissolving an appropriate amount of concentrated acid (Merck, Darmstadt, Germany) in distilled water. 2-(2-Benzothiazolylazo)-4-methoxy-phenol (BTAMP) was synthesized according to the method described previously.30 Stock solution of 5 × 10−3 M was prepared by dissolving an appropriate weight of the pure reagent in the minimum amount of ethanol (15 mL) and then diluting to the mark in a 100 mL measuring flask with ethanol (Merck, Darmstadt, Germany).Solutions of alkali metal salts (1.0%) and various metal salts (0.1%) (Sigma, St Louis, MO, USA) were used to study the interference of anions and cations, respectively. Acetate buffer solutions (HOAc–NH4OAc buffer) of pH 2.75–6.11 were prepared as recommended.31
Preparation of the amberlite XAD-16 column loaded with BTAMP
Amberlite XAD-16 (Merck, Darmstadt, Germany) was treated with an ethanol–sulfuric acid–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution overnight. Later, the resin was rinsed with deionized water until supernant water (5.0 mL pH 5.5 solution). The packing of the column must be done using ethanol as the eluent since water makes the resin beads float. The resin was saturated with the reagent by passing 2.5 mL of 5 × 10−3 M BTAMP solution in ethanol and 2.0 mL of 5.0% Triton X-114 solution (Sigma, St Louis, MO, USA) at a flow rate of 0.5 mL min−1. Later it was washed with water until the reagent excess was eliminated from the resin. All experiments were carried out in a funnel-tipped glass tube (60 mm × 6 mm) that was used as a column for preconcentration. It was plugged with glass wool and then filled with the XAD-16 to the height of 1.0–1.5 cm. Before sample loading the column must be preconditioned by passing a sulfuric acid solution.
1) solution overnight. Later, the resin was rinsed with deionized water until supernant water (5.0 mL pH 5.5 solution). The packing of the column must be done using ethanol as the eluent since water makes the resin beads float. The resin was saturated with the reagent by passing 2.5 mL of 5 × 10−3 M BTAMP solution in ethanol and 2.0 mL of 5.0% Triton X-114 solution (Sigma, St Louis, MO, USA) at a flow rate of 0.5 mL min−1. Later it was washed with water until the reagent excess was eliminated from the resin. All experiments were carried out in a funnel-tipped glass tube (60 mm × 6 mm) that was used as a column for preconcentration. It was plugged with glass wool and then filled with the XAD-16 to the height of 1.0–1.5 cm. Before sample loading the column must be preconditioned by passing a sulfuric acid solution.
Procedure for the sorption of boron on the column
An aliquot of boron solution (containing 0.25–625 ng) was placed in a 50 mL beaker containing 5.0 mL of acetate buffer solution of pH 5.5, which was then diluted to 25 mL with bidistilled water. This solution was passed through the column at a flow rate of 2.0 mL min−1. After passing this solution, the column was eluted with 5.0 mL of deionized water. The adsorbed boron complex on the column was eluted with 4.0 mL of 2.0 M sulfuric acid solution at a flow rate of 1.0 mL min−1. The eluent was collected in a 5.0 mL measuring flask and diluted to the mark with 2.0 M sulfuric acid, and boron was determined spectrophotometrically at λmax 622 nm against a reagent blank similarly treated.Reference method (ICP-AES method)
The measurements were carried out with an ICP-AES Perkin Elmer model 5300 DV. The operation conditions for ICP-AES were as follows: emission line: B(I) 249.773 nm (other lines, 208.893, 208.959, 249.678 nm were also used to confirm the results in analyses), plasma power supply: 1.0 kW, observation height: 6.0 mm, plasma gas flow: 10 L min−1, auxiliary gas flow: 0.5 L min−1, nebuliser gas flow: 0.6 L min−1, photomultiplier voltage: 600 V, sample uptake rate: 1.7 mL min−1, integration time: 1.0 s and replicates: 3.Results and discussion
Absorption spectra
The absorption bands of BTAMP and its complex in acidic media are located at 498 nm and at 622 nm, respectively.Reaction conditions
The reaction conditions were investigated with a 100 ng mL−1 solution of boron. Adsorption was carried out in different buffer media, and other variables were kept constant. It was found that the boron complex was quantitatively adsorbed on amberlite XAD-16 resin in an acetate buffer solution of pH 5.5. Addition of 4.0–6.0 mL of a pH 5.5 solution did not affect the retention of boron and the use of 5.0 mL is recommended.The effects of surfactants on the B(III)–BTAMP system were investigated. The results showed that, in the absence of surfactants, anionic or cationic surfactants, the B(III)–BTAMP chromogenic system gives a low absorption, whereas in the presence of nonionic surfactants, the absorption of the chromogenic system increases markedly. Various nonionic surfactants enhance the absorbance in the following sequence: Triton X-114 > Triton X-100 > Tween-80 > Tween-60 > Tween-20 > emulsifier-OP. The Triton X-114 was the best additive, and the use of 1.5–2.5 mL of 5.0% Triton X-114 solution gave a constant and maximum absorbance (Fig. 1). Consequently, the use of 2.0 mL was recommended.
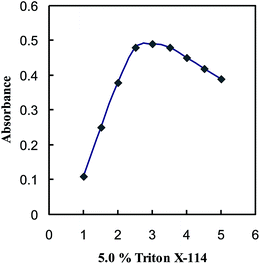 | ||
| Fig. 1 Effect of Triton X-114 on the complexation of 100 ng mL−1 B(III) with 5.0 × 10−4 M BTAMP at the optimum experimental conditions. | ||
For up to 100 ng of B(III), the use of 2.5 mL of 5 × 10−3 M BTAMP solution was found to be sufficient for a complete reaction. Accordingly, 2.5 mL of 5 × 10−3 M BTAMP solution was added in all further measurements.
The flow rate was varied from 0.1 to 5 mL min−1. It was found that a flow rate of 0.1–3.0 mL min−1 did not affect adsorption. A flow rate of 2.0 mL min−1 was recommended in all experiments.
The volume of the aqueous phase was varied in the range of 2.0–100 mL under the optimum conditions, keeping the other variables constant. It was observed that the highest absorbance value was almost constant up to 25 mL. However, for convenience, all the experiments were carried out with 25 mL of the aqueous phase.
Preliminary observations indicated that the boron complex was desorbed completely with 4.0 mL of 2.0 M sulfuric acid. Therefore, 4.0 mL of 2.0 M sulfuric acid was used in the present work.
Sorption capacity of resin for ligand and boron
The sorption capacity of the amberlite XAD-16 resin for ligand and bismuth was also evaluated using the Langmuir model.32 The resin has a sorption capacity of 1.2 and 0.5 mg g−1 of XAD-16 resin for ligand and boron, respectively.Stoichiometric ratio
The nature of the complex was established at the optimum conditions described above using the molar ratio and continuous variation methods. The plot of absorbance versus the molar ratio of BTAMP to B(III), obtained by varying the BTAMP concentration, showed inflection at a molar ratio of 2.0, indicating the presence of two BTAMP molecules in the formed complex. Moreover, the Job method showed a ratio of BTAMP to B(III) = 2.0. Consequently, the results indicated that the stoichiometric ratio was (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) [BTAMP
1) [BTAMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B(III)]. The conditional formation constant (log
B(III)]. The conditional formation constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K), calculated using the Harvey and Manning equation applying the data obtained from the above two methods, was found to be 5.78, whereas the true constant was 5.55.
K), calculated using the Harvey and Manning equation applying the data obtained from the above two methods, was found to be 5.78, whereas the true constant was 5.55.
Effect of foreign ions
Various salts and metal ions were added individually to a solution containing 100 μg of boron and the general procedure was applied. The tolerance limit was set as the concentration of the diverse ion required to cause ±5.0% error in the determination of boron. The tolerance limit (error < 5.0%) is given in Table 2. Among the salts examined, most did not interfere at the gram or milligram level except EDTA which may be due to the high formation constant of the B–EDTA complex over the boron–BTAMP complex. Thus, the method is highly selective and therefore, has been applied successfully to the trace determination of boron in various biological and water samples without any prior separations.| Foreign ion | Tolerance limit/mg | Foreign ion | Tolerance limit/mg |
|---|---|---|---|
| CH3COO− | 750 | Tartrate | 300 |
| Citrate | 225 | Oxalate | 175 |
| K(I) | 500 | Benzoate | 125 |
| Na(I) | 500 | Mo(VI) | 150 |
| Ca(II) | 100 | Cr(III) | 100 |
| Mg(II) | 75 | Al(III) | 75 |
| Zn(II) | 60 | W(VI) | 50 |
| Fe(III) | 50 | V(V) | 30 |
| Ni(II) | 35 | Cl2 | 20 |
| Co(II) | 15 | Ti(IV) | 10 |
| Pb(II) | 25 | I2 | 7.5 |
| Cu(II) | 15 | NO32− | 40 |
| Mn(II) | 10 | SO42− | 100 |
| Cd(II) | 7.5 | SiO32− | 15 |
| Bi(III) | 5.0 | PO42− | 30 |
Calibration curve and sensitivity
The calibration curve showed that the system obeys Beer’s law in the concentration range of 0.5–125 ng B(III) per mL in the measured solution. For more accurate results, the Ringbom optimum concentration range was found to be 0.2–110 ng B(III) per mL in the measured solution. The linear regression equation obtained was A = 0.413C (μg mL−1) + 0.003 (r = 0.9994). The molar absorptivity was calculated to be 4.46 × 105 L mol−1 cm−1 at 622 nm, whereas the Sandell sensitivity was found to be 2.42 ng cm−2. The standard deviations of the absorbance measurements were calculated from a series of 13 blank solutions. The limits of detection (K = 3) and of quantification (K = 10) of the method were established33 and recorded in Table 3, according to the IUPAC definitions (C1 = KSo/s where C1 is the limit of detection, So is the standard error of blank, s is the slope of the standard curve and K is the constant related to the confidence interval. The relative standard deviation was 1.73% obtained from a series of 10 standards each containing 100 ng mL−1 of B(III).| Parameter | Value | Parameter | Value |
|---|---|---|---|
| a A = a + bC, where C is the concentration of iron in ng mL−1. | |||
| Beer’s law limit (ng mL−1) | 0.5–125 | Regression equationa | |
| Ringbom optimum range (ng mL−1) | 0.10–110 | Slope (b) | 0.413 |
| Molar absorptivity (L mol−1 cm−1) | 4.46 × 105 | Intercept (a) | 0.03 |
| Sandell sensitivity (ng cm−2) | 2.42 | Correlation coefficient (r) | 0.9994 |
| Detection limit (ng mL−1) | 0.15 | RSD (%) | 1.73 |
| Quantification limit (ng mL−1) | 0.49 | Stoichiometric ratio (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M) M) |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
The sensitivity expressed as molar absorptivity of the proposed method was compared with that of published spectrophotometric methods. The higher sensitivity of the proposed method is notable, greater even than that of other methods14–25 based on spectrophotometry. Although the measurable concentration range is approximately 5.0–150 ng L−1 with ICP-AES, the proposed method was very simple and accurate.
Analysis of boron in biological materials and water samples
The accuracy and applicability of the proposed method has been applied to the determination of boron in National Institute for Environmental Studies (NIES) no. 5 human hair; and NIES no. 7 tea leaves. A 0.1 g sample was taken in a beaker and dissolved in concentrated nitric acid (∼5.0 mL) with heating. The solution was cooled, diluted and filtered. Since no standard biological sample containing boron was available, the experiment was conducted by adding a known amount of boron to the standard biological sample. The filtrate was made up to 50 mL with water in a calibrated flask. An aliquot (5.0–25 mL) of the sample solution was taken individually and boron was determined by the general procedure. The results are given in Table 4.| Samplea | Certified composition of the boron added (μg) to reference samples | Foundb after addition of boron |
|---|---|---|
| a NIES: National Institute of Environmental studies reference materials.b Average of five determinations ± standard deviation.c No boron was present in these standard samples. Therefore, boron was added from the standard solution in each case. | ||
| NIE, no. 5 human hair | Pb, 6.0; Cd, 0.20; K, 34; Rb, 0.19; Sb, 0.07; Zn, 169; Al, 240; Fe, 225; Mg, 208; Hg, 4.4; Sc, 0.05; Se, 1.4; Na, 26; Sr, 2.3; Ti, 2.3; Ca, 728; Cr, 104; Ba, 2.2; Co, 0.1; Mn, 5.2; Cu, 16.3; Ni, 1.8 μg g−1; Bc added 1.0 μg | 0.997 ± 0.02 |
| NIES, no. 7 tea leaves | Pb, 0.80; Cd, 0.030; Sb, 0.014; Zn, 33; Cr, 0.15; Al, 775; Mg, 1530; Ba, 5.7; K, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600; Sc, 0.011; Na, 15.5; Sr, 3.7; Ca, 3200; Cs, 0.22; Co, 0.12; Mn, 7.0; Ni, 6.5; Cu, 7.0 μg g−1; Bc added 0.75 μg 600; Sc, 0.011; Na, 15.5; Sr, 3.7; Ca, 3200; Cs, 0.22; Co, 0.12; Mn, 7.0; Ni, 6.5; Cu, 7.0 μg g−1; Bc added 0.75 μg |
0.745 ± 0.07 |
In order to confirm the applicability of the proposed method, it has been applied to the determination of microgram amounts of boron in real water samples. For water samples, the samples were acidified with sulfuric acid and filtrated through a 0.45 μm filter. The boron content was analyzed according to the general procedure. An ICP-AES method was used as a reference method and the results are shown in Table 5.
| Sample | Boron (ng mL−1) | t-testb | F-valueb | ICP-AES (ng mL−1) | |
|---|---|---|---|---|---|
| Added | Founda | ||||
| a Average of four determinations ± standard deviation.b Theoretical values for t and F at 95% confidence limit (n = 5) were 2.57 and 5.05, respectively. | |||||
| Potable water | — | 41.1 ± 2.1 | — | 40.0 ± 1.2 | |
| 50 | 145.9 ± 1.9 | 1.38 | |||
| 100 | 142.4 ± 2.1 | 2.76 | |||
| Well water | 280.3 ± 2.1 | 267 ± 2.5 | |||
| 150 | 431.2 ± 1.4 | 3.23 | |||
| 300 | 583.8 ± 0.8 | 1.56 | |||
| River Nile water | — | 133.5 ± 1.4 | 136.2 ± 2.2 | ||
| 175 | 304.3 ± 1.1 | 1.63 | |||
| 350 | 482.6 ± 1.5 | 2.87 | |||
| Rain water | — | 28.7 ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
30.1 ± 2.1 | ||
| 75 | 104.4 ± 1.1 | 3.11 | |||
| 150 | 177.6 ± 0.8 | 1.52 | |||
| Mineral water | — | 33.0 ± 1.4 | 34.5 ± 1.7 | ||
| 50 | 84.1 ± 1.3 | 1.47 | |||
| 100 | 134.8 ± 2.6 | 2.93 | |||
| Tap water | — | 88.5 ± 1.6 | 90.4 ± 2.1 | ||
| 200 | 287.4 ± 1.0 | 1.72 | |||
| 400 | 488.2 ± 1.3 | 3.48 | |||
| Sea water | — | 247.8 ± 0.7 | 242.8 ± 1.8 | ||
| 125 | 273.7 ± 0.9 | 2.74 | |||
| 250 | 499.4 ± 1.0 | 1.34 | |||
The performance of the proposed method was assessed by calculation of the t-value (for accuracy) and F-test (for precision) compared with the ICP-AES method.7 The mean values were obtained in a Student’s t- and F-tests at 95% confidence limits for five degrees of freedom.34 The results showed that the calculated values (Table 5) did not exceed the theoretical values. The wider range of determination, higher accuracy, increased stability and lower time consumption show the advantages of the proposed method over the other method.
Application to the determination of boron in ceramic materials
Ceramic raw materials were dried at 40 °C for 12 h and finely ground to pass completely through a 200 mesh. A total of 0.1 g of sample was weighed and transferred into a 250 mL vessel, and 20 mL of 10% HCl was added. The mixture was boiled for 1.0 min, then left in a thermostatically controlled bath for 1.0 h at 70–80 °C. Subsequently, the sample solution was filtered through a Whatman no. 40 filter-paper and neutralized with 1.0 M NaOH to a pH of about 7.0 (using a pH-meter). Finally, the solution was diluted to 100 mL with de-ionized water. The ceramic frits and pigments were dried at 110 °C and finely ground to pass completely through a 200 mesh. A total of 0.1 g of sample was mixed in a platinum crucible with a 7-fold amount of Na2CO3–ZnO (2 + 1). The mixture was heated and placed in a muffle furnace at 900 °C for 10 min. The sintered samples were transferred into a porcelain capsule and 50 mL of hot de-ionized water was added. The mixture was filtered through a Whatman no. 40 filter-paper and centrifuged if necessary. The resulting solution was neutralised with 1.0 M NaOH to a pH of about 7.0 (using a pH-meter), then diluted to 100 mL with de-ionized water. The sample solution, containing not more than 30 mg of boron was transferred into a 25 mL calibrated flask and the boron concentration was determined as described in the general procedure. The results are shown in Table 6.| Sample | Proposed methoda | ICP-AESa (ng mL−1) |
|---|---|---|
| a Results expressed as X ± st/n1/2, where X is the mean of n observations of x, s is the standard deviation and t is the distribution value chosen for the desired confidence level. | ||
| 1 | 1.17 ± 0.01 | 1.14 ± 0.02 |
| 2 | 1.35 ± 0.07 | 1.39 ± 0.05 |
| 3 | 1.52 ± 0.10 | 1.58 ± 0.11 |
| 4 | 2.75 ± 0.02 | 2.72 ± 0.04 |
| 5 | 2.94 ± 0.05 | 2.99 ± 0.07 |
| 6 | 3.71 ± 0.02 | 3.76 ± 0.03 |
| 7 | 4.41 ± 0.02 | 4.43 ± 0.08 |
| 8 | 5.34 ± 0.12 | 5.29 ± 0.17 |
Conclusions
The proposed method has several advantages. Firstly, BTAMP is one of the most easily prepared high purity, sensitive, and selective spectrophotometric reagents for boron determination. The molar absorptivity was found to be up to 4.46 × 105 L mol−1 cm−1 at 622 nm in the measured solution. The higher sensitivity of the proposed method is notable, greater even than other methods based on spectrophotometry. Secondly, the detection and quantification limits, are 15 and 49.5 ng mL−1 in the original sample. Thirdly, most common ions do not interfere with the determination suggesting the high selectivity of the proposed method. Fourthly, successful application of the proposed method to the determination of low levels of boron in biological and water samples was carried out with good results. Finally, the proposed method is simple and more sensitive at the nanogram level than other methods commonly used, in addition to having lower tolerance limits.References
- Agency for toxicological substances and diseases registry, July 1992, Toxicological profile for boron, http://www.atsdr.cdc.gov/toxprofiles/tp26.html, 2005/09/02.
- World Health Organization, Guidelines for Drinking Water Quality, Geneva, 2nd edn, 1998, vol. 1 Search PubMed.
- R. N. Sah and P. H. Brown, Microchem. J., 1997, 56, 285 CrossRef CAS.
- M. Burguera, J. L. Burguera, C. Rondon and P. Carrero, Spectrochim. Acta, Part B, 2001, 56, 845 Search PubMed.
- D. Y. Sarıca and N. Ertaş, Turk. J. Chem., 2001, 25, 305 Search PubMed.
- D. H. Sun, J. K. Waters and T. P. Mawhinney, J. Anal. At. Spectrom., 1997, 12, 675 RSC.
- T. U. Probst, N. G. Berryman, P. Lemmen, L. Weissfloch, T. Auberger, D. Gabel, J. Carlsson and B. Larsson, J. Anal. At. Spectrom., 1997, 10, 1115 RSC.
- T. Wilke, H. Wildner and G. Wünsch, J. Anal. At. Spectrom., 1997, 9, 1083 RSC.
- D. H. Sun, R. L. Ma, C. W. McLeod, X. R. Wang and A. G. Cox, J. Anal. At. Spectrom., 2000, 15, 257 RSC.
- A. S. Al-Ammar, R. K. Gupta and R. M. Barnes, Spectrochim. Acta, Part B, 2000, 55, 629 CrossRef.
- S. Kozono, S. Takahashi and H. Haraguchi, Analyst, 2002, 127, 930 RSC.
- C. J. Park and S. Song, J. Anal. At. Spectrom., 2003, 18, 1248 RSC.
- S. S. Ramos, F. B. Reig, J. V. G. Adelantado, D. J. Y. Marco, A. D. Carbo and J. A. B. Perez, Spectrochim. Acta, Part B, 2000, 55, 1669 CrossRef.
- J. Pan, Y. Chen and H. Yan, Shanghai Science and Technology Press, Shanghai, 1981, p. 397.
- I. L. Garcia, M. H. Cordoba and C. Sanchez-Pedreno, Analyst, 1985, 110, 1259 RSC.
- D. Chen, F. Lazaro, M. D. Luque de Castro and M. Valcarcel, Anal. Chim. Acta, 1989, 226, 221 CrossRef CAS.
- A. A. Alwarthan, S. S. Alshowiman, S. A. Altamrah and A. A. Baosman, J. AOAC Int., 1993, 76, 601 CAS.
- M. Saito, F. Hirose and H. Okochi, Anal. Sci., 1995, 11, 695 CrossRef CAS.
- K. Nose and M. Zenki, Analyst, 1991, 116, 711 RSC.
- J. Ciba and A. Chrusciel, Fresenius’ J. Anal. Chem., 1992, 342, 147 CrossRef CAS.
- M. Zenki, K. Nose and K. Toei, Fresenius’ J. Anal. Chem., 1989, 334, 238 CrossRef CAS.
- L. Zaijun, Y. Yuling, P. Jiaomai and T. Jan, Analyst, 2001, 126, 1160 RSC.
- C. D. A. Tumang, G. C. D. Luca, R. N. Fernandes, B. F. Reis and F. J. Krug, Anal. Chim. Acta, 1998, 374, 53 CrossRef CAS.
- A. Hanay, R. Boncukoğlu, M. M. Kocakerim and A. E. Yılmaz, Fresenius Environ. Bull., 2003, 12, 1190 CAS.
- N. Öztürk and D. Kavak, Fresenius Environ. Bull., 2003, 12, 1450 Search PubMed.
- M. Soylak, A. U. Karatepe, L. Elçi and M. Dogan, Turk. J. Chem., 2003, 27, 235 CAS.
- L. Elci, M. Soylak and M. Dogan, Fresenius. J. Anal. Chem., 1992, 342, 175 CrossRef CAS.
- V. N. Bulut, A. Gundogdu, C. Duran, H. B. Senturk, M. Soylak, L. Elci and M. Tufekci, J. Hazard. Mater., 2007, 146, 155 CrossRef CAS PubMed.
- M. Soylak, L. Eici and M. Dogan, J. Trace Microprobe Tech., 2001, 19, 329 CrossRef CAS PubMed.
- A. S. Amin and E. H. El-Mossalamy, J. Trace Microprobe Tech., 2003, 21, 637 CrossRef CAS PubMed.
- H. T. S. Britton, Hydrogen Ions, Chapman and Hall, London, 4th edn, 1952 Search PubMed.
- D. L. Sparks, Environmental soil chemistry, Academic Press, New York, 1995 Search PubMed.
- IUPAC, Spectrochim. Acta, Part B, 1978, 33, 241 CrossRef.
- J. N. Miller and J. C. Miller, Statistics and Chemometrics for Analytical Chemistry, Prentice-Hall, London, 5th edn, 2005 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |
