Highly transparent poly(2-ethyl-2-oxazoline)-TiO2 nanocomposite coatings for the conservation of matte painted artworks†
Abstract
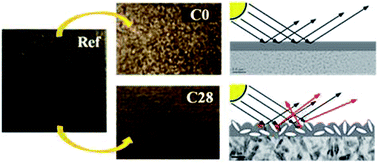
A nanocomposite coating based on TiO2 nanoparticles and poly(2-ethyl-2-oxazoline) is used as consolidant of matte painted surfaces (temperas, watercolors, modern paintings). The aim of this work is to provide advances in the conservation of these works of art, while preserving their optical appearance, in terms of colour and gloss. Fiber Optic Reflectance Spectroscopy (FORS) measurements of a painting-model (an acrylic black monochrome) treated with the nanocomposite coatings revealed that it is possible to match the optical appearance of the painted surface by tuning the amount of nanoparticles in the polymeric matrix. The requirement of retreatability of the material has been verified by removing the nanocomposite cast on the painted surface with aqueous solutions. FTIR and SEM/EDX measurements showed that almost no traces of the nanocomposite remained on the painted surface, allowing its use for the treatment of real paintings. Test were performed using a contemporary studio-model on canvas attributed to Agostino Bonalumi (1935–2013).

 Please wait while we load your content...
Please wait while we load your content...