Facile nanoparticle dispersion detection in energetic composites by rare earth doped in metal oxide nanostructures†
Robert E. Drapera,
David L. Reida,
Tamil S. Sakthivela,
Thomas Sammetb,
Andrew Demkob,
Eric L. Petersenb and
Sudipta Seal*a
aAdvanced Materials Processing and Analysis Center, Nanoscience Technology Center, Materials Science and Engineering, University of Central Florida, Orlando, FL 32816, USA. E-mail: Sudipta.Seal@ucf.edu
bDepartment of Mechanical Engineering, Texas A&M University, College Station, Texas, USA
First published on 4th August 2015
Abstract
The segregation and agglomeration of nanoparticles dispersed in polymer matrices play important roles in nanocomposite performance. A method of rapid and simultaneous visualization of macroscopic and submicron particle dispersion properties is presented, based on nanoparticle luminescence induced by europium doping. The luminescence intensity of polymer composites containing Eu-doped TiO2 nanoparticle catalysts varied with the nanoparticle agglomerate size between 90 nm and 10 μm, and with concentration variations from segregation. These variations were detected by photoluminescence spectroscopy and visible-light photography, making this a facile characterization method for bulk composites without affecting the nanoparticle performance.
1. Introduction
Nanoparticle-polymer composites have shown a myriad of interesting mechanical,1 electrical,2–6 optical,7,8 thermal,9 and energetic properties.10 The quality of the particle dispersion plays a major role in the manifestation of these properties.11,12 In the authors' previous work on polymer-matrix nanocomposite energetic materials, the extent of nanoparticle dispersion was found to have a dramatic effect on the material performance.13 Methods for determining particle dispersion properties are therefore necessary for the practical use of nanoparticles in polymer-matrix composites, especially in the case of manufacturing quality control. Conventional dispersion characterization methods include microscopic inspection, impregnation of inert tracer materials, and flow modeling, each of which suffer from significant drawbacks.One of the most common methods of inspecting particle dispersion, agglomeration, and size in a solid is to perform microscopic analysis on samples taken from different regions in the bulk sample. This is a destructive analysis method that is time intensive, expensive, and difficult to automate. In the case of fine nanoparticle dispersions, specimens must be prepared for transmission electron microscopy, further increasing the time, difficulty, and cost of the process. Quality control techniques have been proposed to use free interface capturing flow models as well as X-ray based measurement techniques.14 The complex flow parameters of granular pastes and the number of different constituents in energetic composites make modeling a practical difficulty.15,16 X-ray, microscopic, and spectroscopic methods require sensitive equipment, are time-consuming processes, and are limited to analyzing only small sections of the composite at a time. Sammet et al. recently demonstrated the use of optical/photographic methods to detect settling and segregation of fluorescing quantum dots in composite energetic materials;17 however, many nanoparticles, including the performance-enhancing particles used in energetic materials, are not intrinsically luminescent. As such, the authors have utilized a method of inducing luminescence in metal-oxide nanoparticles to assess their dispersion in polymer-matrix composites. By inducing luminescence, the particle settling and polydispersion can be measured in a non-destructive way throughout bulk composites using a simple photographic visible light method.
In this paper, we present a general, facile, visible-light method utilizing consumer-grade digital photography to characterize the dispersion properties of metal-oxide nanoparticles in a polymer matrix by doping with luminescent europium. Particle settling and segregation, as well as the extent of nanoparticle agglomeration, are monitored by measuring changes in luminosity intensity, as measured by digital imaging analysis or visible light spectroscopy. This method is therefore capable of rapid and simultaneous measurement of both macroscopic and submicron dispersion properties of nanoparticle-polymer composites. Three-dimensional mapping of the particle dispersion by confocal microscopy is also presented. Titanium dioxide has been chosen as a model nanoparticle system to demonstrate this method. TiO2 nanoparticles have recently been shown to be effective catalysts for energetic composites,10,17–19 and the TiO2 lattice provides an efficient energy transfer network for luminescence.20,21 Hydroxyl-terminated polybutadiene (HTPB) was selected as the polymer matrix, for its use as a binder in composite solid propellants. The developed characterization method has the ability to reduce the cost and time investment required to perform quality control on polymers containing dispersed nanoparticles. The method requires only inexpensive equipment, and can be automated using digital image processing techniques. For the energetic composites of interest in this study, the performance of the nanoparticles is shown to be unaffected by the doping process.
2. Experimental details
2.1. Nanoparticle synthesis
TiO2 nanoparticles containing 5% Eu were prepared using a sol–gel synthesis from a titanium tetraisopropoxide (TIP) precursor and a europium nitrate dopant. In a round bottom reaction vessel with an affixed reflux condenser, ethanol and deionized water were added in equal proportions, along with a small amount of 1 N nitric acid, and were heated to its boiling point. Under aggressive stirring, TIP was added dropwise into the solution, where it started to precipitate. After four hours of reacting, europium nitrate pentahydrate was dissolved in a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ethanol/water mixture and added to the solution. The solution was allowed to reflux for 24 hours, after which the ethanol was distilled off, and the solution was subsequently neutralized, centrifuged, and washed with DI water. The synthesized nanoparticle suspensions were either used as is, or were spray dried to assemble the nanoparticles into spheroidal agglomerates of different controlled sizes. The nanoparticle agglomerate size affects both the catalytic activity and the mixing/settling behavior in the polymer binder. In this experiment, the nanoparticles were prepared in three different agglomerate sizes. Small agglomerates (EuTiO2-Sm: ∼90 nm) were obtained directly from the colloidal suspension; medium (EuTiO2-Md: ∼3 μm), and large (EuTiO2-Lg: ∼10 μm) agglomerates were obtained by spray-drying suspensions of different concentrations using a Bucci B-290 spray drier with an ultrasonic nozzle (see ESI Fig. S1†). The spray-dried powders were either heat-treated (EuTiO2 & EuTiO2-H) at 400 °C for 3 hours, or were used as is. As controls, undoped TiO2 samples were prepared using the same methods (TiO2 & TiO2-H). A list of all prepared samples and affiliated information is presented in Table 1.
50 ethanol/water mixture and added to the solution. The solution was allowed to reflux for 24 hours, after which the ethanol was distilled off, and the solution was subsequently neutralized, centrifuged, and washed with DI water. The synthesized nanoparticle suspensions were either used as is, or were spray dried to assemble the nanoparticles into spheroidal agglomerates of different controlled sizes. The nanoparticle agglomerate size affects both the catalytic activity and the mixing/settling behavior in the polymer binder. In this experiment, the nanoparticles were prepared in three different agglomerate sizes. Small agglomerates (EuTiO2-Sm: ∼90 nm) were obtained directly from the colloidal suspension; medium (EuTiO2-Md: ∼3 μm), and large (EuTiO2-Lg: ∼10 μm) agglomerates were obtained by spray-drying suspensions of different concentrations using a Bucci B-290 spray drier with an ultrasonic nozzle (see ESI Fig. S1†). The spray-dried powders were either heat-treated (EuTiO2 & EuTiO2-H) at 400 °C for 3 hours, or were used as is. As controls, undoped TiO2 samples were prepared using the same methods (TiO2 & TiO2-H). A list of all prepared samples and affiliated information is presented in Table 1.
| Abbreviation | Europium content | Heat treatment | Particle size (nm) | Agglomerate size (μm) |
|---|---|---|---|---|
| TiO2 | None | None | 5 | 1.7–5.1 |
| TiO2–H | None | 400 °C | 7 | 1.8–5.1 |
| EuTiO2 | 5% Eu | None | 5 | 2.2–10.5 |
| EuTiO2-H | 5% Eu | 400 °C | 5 | 2.3–10.2 |
| EuTiO2-Sm | 5% Eu | None | 5 | 0.02–0.16 |
| EuTiO2-Md | 5% Eu | None | 5 | 1.4–8.5 |
| EuTiO2-Lg | 5% Eu | None | 5 | 3.8–22.8 |
2.2. Nanoparticle-polymer mixing
The nanoparticles in the forms of aqueous suspensions and spray-dried powders were dispersed in HTPB resin, which is a high viscosity liquid. The powders were directly mixed with the HTPB by stirring by hand. The aqueous nanoparticle suspensions were wet-mixed into the HTPB. To mix the suspension into the HTPB, the concentration of the suspension was measured using thermogravimetric analysis. After determining the concentration, the appropriate amount of suspension was added to a beaker containing HTPB stirred at 500 rpm at 50 °C. Due to the insolubility of HTPB in water, the particle suspension remains immiscible with HTPB during the mixing process, but the particles become embedded in the polymer over time.2.3. Composite propellant preparation
To create samples that were accurate representations of propellant formulations, the catalyst embedded HTPB was mixed with a common solid propellant oxidizer, ammonium perchlorate (AP). The AP was mixed in a commercial standard 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 weight proportion with the HTPB by adding the oxidizer crystals into the liquid HTPB binder with embedded catalysts, and mixed together using the hand mixing methods described in Stephens et al.,18 forming a granular paste. Once the oxidizer crystals were mixed into the liquid HTPB, the AP/HTPB paste was cross-linked by thoroughly mixing in isophorone diisocyanate (IPDI) in a proportion of 10
20 weight proportion with the HTPB by adding the oxidizer crystals into the liquid HTPB binder with embedded catalysts, and mixed together using the hand mixing methods described in Stephens et al.,18 forming a granular paste. Once the oxidizer crystals were mixed into the liquid HTPB, the AP/HTPB paste was cross-linked by thoroughly mixing in isophorone diisocyanate (IPDI) in a proportion of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05 by weight, and the samples were vacuumed at room temperature to remove any bubbles or air pockets within the liquid rubber. Once the samples were free of bubbles and irregularities, the paste was poured into 6.04 mm diameter strands of PTFE tubing to cure at 63 °C for one week. These strands were used for the photographic fluorescence measurements, as well as the microscopy characterization used to justify the photographic measurements, and the burning studies to investigate the effect of the dopant on the catalytic activity of the nanoparticles.
1.05 by weight, and the samples were vacuumed at room temperature to remove any bubbles or air pockets within the liquid rubber. Once the samples were free of bubbles and irregularities, the paste was poured into 6.04 mm diameter strands of PTFE tubing to cure at 63 °C for one week. These strands were used for the photographic fluorescence measurements, as well as the microscopy characterization used to justify the photographic measurements, and the burning studies to investigate the effect of the dopant on the catalytic activity of the nanoparticles.
To test the burning profile of the samples, the cured strands were mounted in a vertical high-pressure bomb reactor. Burn inhibitor was applied to the sides of the propellant strands to ensure that the samples burned linearly down the cylindrical strand. The strands were ignited using a nichrome wire at one end of the sample, while the emission spectra and pressure changes were recorded to find the combustion rate of the samples.
2.4. Sampling methodology and measurement
To characterize the titania particles as accurate representations of the particles in the solid propellant, microscopic analysis was performed on the particles while embedded in the HTPB binder. To obtain sections of appropriate thickness for electron microscopy, and confocal microscopy, a Leica ultra-cryomicrotome with a diamond blade was used to generate sections ranging from 10 nm to 15 μm. Sections were cut at −120 °C to decrease the toughness, allowing for low roughness cuts of the polymer composites. The lower temperature also promoted greater particle adhesion to the binder during cutting, decreasing particle pull out; when coupled with the ultra-sharp diamond knife, the agglomerate particles were allowed to be sectioned.X-ray diffraction (XRD) was performed with a Rigaku D/MAX diffractometer and a copper X-ray source. X-ray photoelectron spectroscopy (XPS) was performed with a PHI Quantum 2000 XPS with a monochromatic Al K X-ray beam. Scanning electron microscopy (SEM) was performed with a Zeiss Ultra-55 FEG SEM. All SEM samples were sputtered with gold-palladium prior to imaging. High resolution transmission electron microscopy (HRTEM) was performed with a Tecnai F30 TEM at 300 kV. Confocal microscopy was performed with a Nikon A1Rsi spectral imaging confocal system. UV-visible spectroscopy (UV/vis) was performed with a PerkinElmer Lambda 740S UV/vis spectrometer with a diffuse reflectance accessory for the powder samples. Photoluminescence measurements (PL) were performed using a Hitachi F-7000 fluorescence spectrophotometer.
The luminescent nanoparticles allow for multiple ways of measuring the particle dispersion, including conventional invasive methods, as well as new spectroscopic and microscopic procedures. However, one of the advantages of the proposed dispersion measurement procedure is that information on particle settling and segregation can be detected in bulk using inexpensive methods and equipment. To provide visible light measurements of the particle dispersion, a shrouded photographic setup was constructed. A 100 W Mercury Arc Bulb supplies the initial polychromatic light for the sample excitation. The light source is connected to a shroud containing the sample to prevent ambient light from affecting the images and measurements. The light from the mercury bulb sequentially passes through a convex smoothing lens, and a blue excitation band pass filter, emitting 470 nm light. This light is sufficiently close to the 464 excitation peak of the EuTiO2 nanoparticles, causing the strand with embedded EuTiO2 particles to fluoresce. The fluorescing light radiating from the sample passes through a red band pass filter sufficiently close to the 615 nm emission peak of the EuTiO2 particle, where it can be simply photographed using a DSLR camera. A schematic of this experimental setup is shown in Fig. 1.
3. Results and discussion
3.1. Particle luminescence
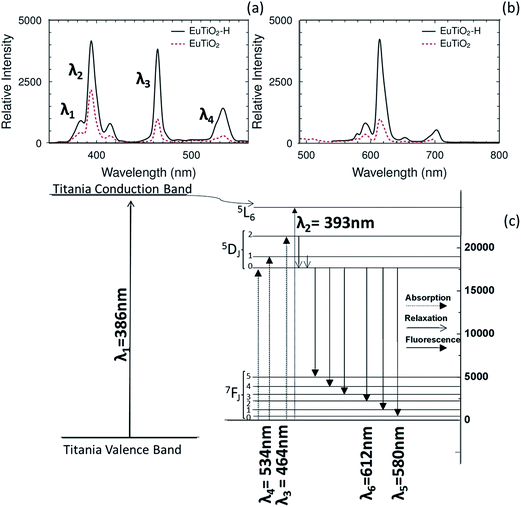
The photoluminescence excitation and emission spectra of the EuTiO2 agglomerate powders are shown in Fig. 2, and closely match the standard orbital transitions expected from europium oxide. Areas of locally maximum absorption in photoluminescence spectroscopy correspond to excitation transitions and give corresponding emission spectra of the agglomerate particles, as shown in Fig. 2b. These distinct absorption peaks corroborate the existence of excitation at 393 nm, 464 nm, and 534 nm and likely correspond to europium 7F0 → 5L6, 7F0 → 5D1, and 7F0 → 5D0 transitions, respectively.20,22–24 There is also a broad excitation from the valence band to the conduction band in the titania host matrix, positioned around 386 nm (3.2 eV), which allows electrons to be excited in a broader range of UV light, and to subsequently relax through defect states into the elevated band of europium. Fig. 2b shows that the particles have a sharp, narrow emission peak at 615 nm. This peak corresponds closely to an electronic transition from the 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 374 cm−1 state in the 5D0 orbital to the 1036 cm−1 state in the 7F2 orbital of molecular europium.25–27 This single-molecule transition corresponds to a 612 nm emission, but when the transition occurs from an energy band in the solid state, the emission is typically shifted to a value of 615 nm.28,29 This particular transition is amplified relative to the other orbital states through coordination, bonding, or complexion to host materials through lattice substitution, ligands, or other methods of bonding to secondary materials.30 The UV excitation at 393 nm was more intense than the near-UV peak at 464 nm, but both caused emission in the visible red range at 615 nm. For UV-vis spectroscopy of the aforementioned regions, see ESI Fig. S2.† The heat-treated material, relative to the un-heat treated one, had a greater intensity of absorption and emission at all peaks. This indicates that there is a greater excitability of electrons from the energy bands of the host titania and europium dopant, and an increased luminescence at all emission energies resulting from these host and dopant excitations.31–33 This increase in luminescence after heat treatment is often seen in the fluorescence of lanthanide doped materials as a result of defect reduction, elimination of dangling bonds, and lattice strain reduction. These lattice modifications increase the excitation and emission intensity of lanthanides in host matrices by increasing the efficiency of energy transfer to the emitting atoms.34,35
374 cm−1 state in the 5D0 orbital to the 1036 cm−1 state in the 7F2 orbital of molecular europium.25–27 This single-molecule transition corresponds to a 612 nm emission, but when the transition occurs from an energy band in the solid state, the emission is typically shifted to a value of 615 nm.28,29 This particular transition is amplified relative to the other orbital states through coordination, bonding, or complexion to host materials through lattice substitution, ligands, or other methods of bonding to secondary materials.30 The UV excitation at 393 nm was more intense than the near-UV peak at 464 nm, but both caused emission in the visible red range at 615 nm. For UV-vis spectroscopy of the aforementioned regions, see ESI Fig. S2.† The heat-treated material, relative to the un-heat treated one, had a greater intensity of absorption and emission at all peaks. This indicates that there is a greater excitability of electrons from the energy bands of the host titania and europium dopant, and an increased luminescence at all emission energies resulting from these host and dopant excitations.31–33 This increase in luminescence after heat treatment is often seen in the fluorescence of lanthanide doped materials as a result of defect reduction, elimination of dangling bonds, and lattice strain reduction. These lattice modifications increase the excitation and emission intensity of lanthanides in host matrices by increasing the efficiency of energy transfer to the emitting atoms.34,35
Nanoparticles of 3 different agglomerate sizes (90 nm, 3 μm, 10 μm) were dispersed in HTPB, and the photoluminescence intensities of emission at 580 nm and 615 nm are plotted in Fig. 3. Emission intensity at both wavelengths was found to increase with decreasing agglomerate size. The dependence of rare earth luminescence intensity with crystallite size has been profiled in several studies,36–38 but the cause of this phenomenon remains debated.34 For materials with dominant quantum confinement effects, there is a decrease in the luminescence with increasing particle size.34,37,39 However, for materials with dominant defect interactions, the luminescence tends to increase with crystallite size.36,40 This increase in luminescence intensity can be attributed to the reduction in grain boundaries, which generally act as quenching points for the luminescent energy. Whether these trends could hold true for agglomerate size, as well as crystallite trends, depends on the material system, with varying literature results being presented.34,38,41 Graeve et al. pointed out the difficulty in assessing this trend due to the inconsistencies in the use of particle, crystallite, and agglomerate in the literature.34 Most studies investigating between europium and doped yttria find that luminescence intensity tends to decrease with increasing agglomerate size due to the higher volume of phosphors at the agglomerate surface.34,38 However, in their study of europium doped yttrium vanadium oxide, Georgescu et al. noted that this trend is partially affected by the effective refractive index, due to its effect on the fluorescence lifetime.41 The complexity of the interconnected factors involved in luminescence indicates that any trends in agglomerate or crystallite size will be highly dependent on the luminescent molecules, and the environment they are in. However, for a given material system, trends in luminescence intensity with agglomeration condition can be observed. Changes in nanoparticle agglomeration result in changes in the luminescence intensity of the bulk composite. In the present system, an increase in nanoparticle agglomerate size lead to decreased composite luminescence. Additionally, we observed a shift in the relative intensity of the orbital transitions with varying polymer-embedded agglomerate size. As the agglomerate size decreased from the micrometer to nanometer range, the 5D0 → 7F0 transition at ∼580 nm luminesces at a greater intensity value relative to the 615 nm 5D0 → 7F2 transition intensity (see ESI Fig. S3† for the PL spectra). This relative peak intensity shift could provide another indication of nanoparticle agglomeration condition in the bulk polymer.
3.2. Crystallite structure and chemistry
XRD crystallite sizes, shown in Fig. 4, indicate no change in crystallite size across the EuTiO2 samples. Un-heat treated EuTiO2 powder shows a primarily anatase titania structure, with evidence of a (121) peak belonging to brookite near 30°. Deconvolution shows that this (121) peak has a slight presence of (222) of europium oxide. However, after heat treating (EuTiO2-H), the peak becomes dominated by the presence of (222) europium oxide. XRD of the non-doped TiO2 powder (TiO2) also exhibits the presence of a brookite (121) peak, which loses intensity after heat treatment (TiO2-H).The TEM micrograph shown in Fig. 5a corroborates the XRD crystallite size calculations shown in Table 1, and reaffirms the assertion that the spherical nanoparticles that make up the varying agglomerate sizes are crystalline, and around 5 nm in size. Selected area electron diffraction (SAED) (Fig. 5b) of the agglomerated nanocrystals shows indexed diffraction rings consistent with the anatase crystal phase.
The spray-dried powders were analyzed by XPS to study the surface elemental composition and chemistry of the samples with and without heat-treatment. The XPS spectra in Fig. 6 show the surface chemistry of the fluorescing europium doped titanium oxide in both the unmodified and heat treated states for the small and large agglomerates. The Ti 2p3/2 peak in the un-heat treated powder was positioned at BE = 458.8 eV, which is characteristic of the binding energy shift of Ti4+ from 458.6 eV, as a result of the formation of Ti–O–Eu bonds. However, the heat-treated samples showed a reduced Ti 2p3/2 binding energy relative to the undoped powder, with a peak at 458.2 eV. The O 1s peak had two components at around 529.7 eV, and 530.8 eV for both the heat treated and un-heat treated powders. These peaks would typically denote surface oxide and hydroxide, respectively, however the 530.8 eV peak is likely also indicative of Eu–O surface bonds, shifted from the peak position of Ti–O bonds.22 As Eu3+ and Ti4+ have a large difference in ionic radius (0.0947 nm to 0.0605 nm, respectively, for six-fold coordination),22 and thus a low solubility, it was originally hypothesized that the heat treating process would allow trapped europium to migrate to the surface of the nanoparticles, and form crystalline europium oxide in clusters on the surface, increasing the surface Eu concentration. However, the surface chemistry data in Fig. 6d shows a lower relative presence of europium to titanium after heat treatment, indicating that the europium diffuses from the surface to the particle interior during heat treatment. It should be noted that higher europium oxide surface content does not necessarily correspond to higher fluorescence intensity, as shown by the PL spectra. Radiationless energy transfer, or cross relaxation due to dopant pair formation, can quench the fluorescence in materials with concentration of fluorescent molecules greater than a critical level, generally between 2–6 mol% for europium.42,34 The current material system has an overall concentration of ∼5% Eu to provide sufficient luminescence without significant quenching effects. However, diffusion or segregation of the dopant in the material could lead to regions or features with higher critical concentration for quenching. This quenching effect in materials in excess of the critical dopant level can be diminished if europium complexes are formed to reduce the vibrational coupling of hydroxide ions. This result could be achieved with organic ligands or covalent complexes that act as antennas to allow increased transmission of absorbed energy to the luminescent ions.43
 | ||
| Fig. 6 (a) Eu 3d XPS spectra. (b) Ti 2p XPS spectra. (c) O 1s XPS spectra. (d) Oxygen and dopant atomic ratios at the particle surfaces. | ||
3.3. Microscopy and dispersion measurement
SEM microscopy was conducted to provide high-magnification images of the dispersed agglomerates within the polymer binder (see Fig. 7 for SEM images of EuTiO2 before and after adding into the HTPB polymer matrix), which confirms the retention of the spherical shape even after mixing in the composite. Samples of the composite were also imaged using fluorescence confocal microscopy to confirm that the observed bulk luminescence of the composite originates from the individual nanoparticle agglomerates. The information gained about the particle dispersion and morphology by SEM is limited due to the small penetration depth into the rubber binder. By contrast, when confocal microscopy is used the particles embedded in the HTPB cross sections can be viewed through the whole sectioned sample. Thirty confocal images of the microtomed composite cross section were taken, with focus at 0.5 μm intervals ranging from the bottom to the top of the sample, and then assembled into a 3D stack to create a virtual representation of the particle dispersion. Laser diodes at 405 nm, 458 nm, and 476 nm were used to view the particles in their encasing rubber binder. This technique allows the particle dispersion throughout the entire 15 μm microtomed section to be observed. For additional reference see (insert video link – ESI†).Based on the photoluminescence spectroscopy data, a visible-light illumination apparatus was developed consisting of a mercury arc lamp, blue and red wavelength band pass optical filters, and a DSLR camera, for photographic imaging of macro-scale composite samples containing the Eu-doped fluorescing nanoparticles. Fluorescence was induced in the particle-embedded HTPB strands according to the illumination setup shown in ESI Fig. S4.† When the particles are properly mixed into the binder, shown in Fig. 8d, it can be seen that the 6.04 mm solid composite propellant strands with the Eu-doped particles exhibit significant, consistent luminescence throughout the sample under UV light. When the particles are intentionally poorly mixed into the binder, the samples show inconsistency, with areas of higher particle loading exhibiting greater luminescence, and areas of lower particle loading exhibiting lesser luminescence (Fig. 8e). Color intensity line scans were performed across the sample, yielding the intensity profiles shown in Fig. 8d and e. The intensity values under sample luminescence can be resolved using digital image processing to find areas at the surface of differing intensity, allowing for both a metric to characterize the nanoparticle dispersion. The areas of high particle loading show a red component value more than three times larger than the areas of low loading.
3.4. Catalytic performance
Pure and Eu-doped TiO2 nanoparticles were tested for use as catalysts in composite solid propellants. The samples for burning were prepared by mixing the spray-dried agglomerates with the HTPB binder using the aforementioned hand-mixing technique, and then mixing in monomodal-sized ammonium perchlorate (AP) crystals with an average size of 200 μm. This produces a granular paste that was poured into the 6.04 mm tubing to form strands consisting of 80.0% AP, 19.5% HTPB, and 0.5% of the nanoparticles. These strands were then burned in a high-pressure strand bomb throughout the mid-level pressure range shown in Fig. 9.The fitted lines of the burning rate's correlation with pressure show that the burning rate of the strands containing the EuTiO2 nanoparticles is nearly identical to the non-fluorescing, pure-TiO2 particles, while both show profiles with a significant increase from the baseline, which contains no additive. This similarity in behavior shows that the EuTiO2 maintains the significant increase in the burning rate provided by the pure-TiO2 catalysts. Despite the introduction of the fluorescing dopant, the EuTiO2 nanoparticles were able to moderate the chemical sub-processes of the decomposition reaction of the propellant similarly enough to produce the same burning rate as the undoped nanoparticles. This indicates that the introduction of the dopant enabled the excitation/emission behavior of the catalytic nanoparticles to change, while leaving the chemical and physical properties of the nanoparticles largely unchanged.
4. Conclusions
The introduction of a luminescent dopant into metal oxide nanoparticles dispersed in polymer composites effectively provides a convenient method to measure or visualize particle dispersion in a polymer binder. In one measurement, dispersion properties of metal-oxide nanoparticles such as agglomeration, and macroscale properties such as particle segregation, can both be effectively detected in bulk using facile visible light methods, as well as through normal spectroscopy. These measurements can be additionally enhanced by using heat treatments to alter or amplify the luminescence profile. By using color intensity values under bulk luminescence compared to a reference value, the segregation of particles can be determined and localized to a particular region in the bulk composite. Using spectroscopic methods such as PL, additional information can be gained about the dispersion or agglomeration state of the fluorescing nano-catalysts by comparing the intensity of emission peaks, particularly those of the 5D0 → 7F2 and 5D0 → 7F0 transitions. Additionally, a method for mapping the three dimensional particle dispersion using confocal microscopy was developed. This method allows for a virtual reconstruction of the true particle positions, including information about particle dispersion and agglomeration. With respect to composite energetic materials, this method allows for an expedient quality control procedure with retention of the reaction behavior and performance of the undoped TiO2 additives.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Funding sources
The authors declare no competing financial interest.Acknowledgements
Funding from a Research Experiences for Teachers supplement to NSF REU Grant Number EEC-1004859. The authors thank Janet Dowding, and Jeff Hatcher and the UCF School of Biomedical Sciences for aiding in the use of the confocal microscope.References
- J. Kao, P. Bai, V. P. Chuang, Z. Jiang, P. Ercius and T. Xu, Nano Lett., 2012, 12, 2610–2618 CrossRef CAS PubMed.
- X. Zhang, Q. He, H. Gu, H. A. Colorado, S. Wei and Z. Guo, ACS Appl. Mater. Interfaces, 2012, 5, 898–910 Search PubMed.
- X. Zhang, O. Alloul, Q. He, J. Zhu, M. J. Verde, Y. Li, S. Wei and Z. Guo, Polymer, 2013, 54, 3594–3604 CrossRef CAS PubMed.
- X. Zhang, O. Alloul, J. Zhu, Q. He, Z. Luo, H. A. Colorado, N. Haldolaarachchige, D. P. Young, T. D. Shen, S. Wei and Z. Guo, RSC Adv., 2013, 3, 9453–9464 RSC.
- X. Zhang, Q. He, H. Gu, S. Wei and Z. Guo, J. Mater. Chem. C, 2013, 1, 2886–2899 RSC.
- X. Zhang, S. Wei, N. Haldolaarachchige, H. A. Colorado, Z. Luo, D. P. Young and Z. Guo, J. Phys. Chem. C, 2012, 116, 15731–15740 CAS.
- J. H. Park, Y. T. Lim, O. O. Park, J. K. Kim, J. Yu and Y. C. Kim, Chem. Mater., 2004, 16, 688–692 CrossRef CAS.
- Z. Guo, S. Wei, B. Shedd, R. Scaffaro, T. Pereira and H. T. Hahn, J. Mater. Chem., 2007, 17, 806–813 RSC.
- Z. Ge, Y. Kang, T. A. Taton, P. V. Braun and D. G. Cahill, Nano Lett., 2005, 5, 531–535 CrossRef CAS PubMed.
- D. L. Reid, A. E. Russo, R. V. Carro, M. A. Stephens, A. R. LePage, T. C. Spalding, E. L. Petersen and S. Seal, Nano Lett., 2007, 7, 2157–2161 CrossRef CAS.
- Y. Feng, H. Zou, M. Tian, L. Zhang and J. Mi, J. Phys. Chem. B, 2012, 116, 13081–13088 CrossRef CAS PubMed.
- J. Liu, Y. Gao, D. Cao, L. Zhang and Z. Guo, Langmuir, 2011, 27, 7926–7933 CrossRef CAS PubMed.
- D. L. Reid, K. R. Kreitz, M. A. Stephens, J. E. S. King, P. Nachimuthu, E. L. Petersen and S. Seal, J. Phys. Chem. C, 2011, 115, 10412–10418 CAS.
- T. Shimada, H. Habu, Y. Seike, S. Ooya, H. Miyachi and M. Ishikawa, Flow Meas. Instrum., 2007, 18, 235–240 CrossRef CAS PubMed.
- S. Rigopoulos, Prog. Energy Combust. Sci., 2010, 36, 412–443 CrossRef PubMed.
- A. S. Mukasyan and A. S. Rogachev, Prog. Energy Combust. Sci., 2008, 34, 377–416 CrossRef CAS PubMed.
- T. E. Sammet, M. A. Stephens, E. L. Petersen and B. A. Corbin, J. Propul. Power, 2010, 26, 987–992 CrossRef CAS.
- M. A. Stephens, E. L. Petersen, R. Carro, D. L. Reid and S. Seal, Propellants, Explos., Pyrotech., 2010, 35, 143–152 CrossRef CAS PubMed.
- K. Kreitz, E. Petersen, D. Reid and S. Seal, Combust. Sci. Technol., 2012, 184, 750–766 CrossRef CAS PubMed.
- K. L. Frindell, M. H. Bartl, M. R. Robinson, G. C. Bazan, A. Popitsch and G. D. Stucky, J. Solid State Chem., 2003, 172, 81–88 CrossRef CAS.
- R. Asapu, V. M. Palla, B. Wang, Z. Guo, R. Sadu and D. H. Chen, J. Photochem. Photobiol., A, 2011, 225, 81–87 CrossRef CAS PubMed.
- J. Xu, Y. Ao, D. Fu and C. Yuan, J. Colloid Interface Sci., 2008, 328, 447–451 CrossRef CAS PubMed.
- S. Yao, C. Sui and Z. Shi, J. Rare Earths, 2011, 29, 929–933 CrossRef CAS.
- Y. Wang, H. Cheng, L. Zhang, Y. Hao, J. Ma and B. Xu, J. Mol. Catal. A: Chem., 2000, 151, 205–216 CrossRef CAS.
- C. Leroy, T. Cardinal, V. Jubera, M. Treguer-Delapierre, R. Backov, C. Boissière, D. Grosso, C. Sanchez, B. Viana and F. Pellé, J. Lumin., 2009, 129, 1641–1645 CrossRef CAS PubMed.
- X. Feng, L. Yang, N. Zhang and Y. Liu, J. Alloys Compd., 2010, 506, 728–733 CrossRef CAS PubMed.
- S. S. Stanimirov, G. B. Hadjichristov and I. K. Petkov, Spectrochim. Acta, Part A, 2007, 67, 1326–1332 CrossRef CAS PubMed.
- C. M. Leroy, H. F. Wang, A. Fargues, T. Cardinal, V. Jubera, M. Treguer-Delapierre, C. Boissière, D. Grosso, C. Sanchez, B. Viana and F. Pelle, Phys. Chem. Chem. Phys., 2011, 13, 11878–11884 RSC.
- Y. Li and B. Yan, J. Mater. Chem., 2011, 21, 8129–8136 RSC.
- G. Plancque, V. Moulin, P. Toulhoat and C. Moulin, Anal. Chim. Acta, 2003, 478, 11–22 CrossRef CAS.
- L. Ying, L. S. Hon, T. White, R. Withers and L. B. Hai, Mater. Trans., 2003, 44, 1328–1332 CrossRef CAS.
- K. Y. Jung, S. B. Park and M. Anpo, J. Photochem. Photobiol., A, 2005, 170, 247–252 CrossRef CAS PubMed.
- O. Berkani, K. Latrous, H. El Hamzaoui, B. Capoen and M. Bouazaoui, J. Lumin., 2012, 132, 2979–2983 CrossRef CAS PubMed.
- O. A. Graeve, S. Varma, G. Rojas-George, D. R. Brown and E. A. Lopez, J. Am. Ceram. Soc., 2006, 89, 926–931 CrossRef CAS PubMed.
- A. Kumar, S. Babu, A. S. Karakoti, A. Schulte and S. Seal, Langmuir, 2009, 25, 10998–11007 CrossRef CAS PubMed.
- J. Mckityrick, B. Hoghooghi, W. Dubbelday, K. Kavanagh, K. Kinsman, L. Shea and E. Sluzky, MRS Proc., 1994, 348, 519 CrossRef.
- E. T. Goldburt, B. Kulkarni, R. N. Bhargava, J. Taylor and M. Libera, J. Lumin., 1997, 72, 190–192 CrossRef.
- J. S. Yoo and J. D. Lee, J. Appl. Phys., 1997, 81, 2810–2813 CrossRef CAS PubMed.
- P. K. Sharma, M. H. Jilavi, R. Nass and H. Schmidt, J. Lumin., 1999, 82, 187–193 CrossRef CAS.
- G. Tessari, M. Bettinelli, A. Speghini, D. Ajo, G. Pozza, L. E. Depero, B. Allieri and L. Sangaletti, Appl. Surf. Sci., 1999, 144, 686–689 CrossRef.
- S. Georgescu, E. Cotoi, A. M. Voiculescu and O. Toma, Rom. Rep. Phys., 2008, 60, 947–955 CAS.
- H. Li, Y. Sheng, H. Zhang, J. Xue, K. Zheng, Q. Huo and H. Zou, Powder Technol., 2011, 212, 372–377 CrossRef CAS PubMed.
- X. L. Wang and B. Yan, Colloid Polym. Sci., 2011, 289, 423–431 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XPS spectra of the powder samples, SEM images of agglomerate powder and size distribution, video of confocal 3-Dimensional stack rotation, UV-visible spectra of powder samples, and additional digital image analysis of bulk samples. See DOI: 10.1039/c5ra10659a |
| This journal is © The Royal Society of Chemistry 2015 |