Graphene: a self-reducing template for synthesis of graphene–nanoparticles hybrids†
Abstract
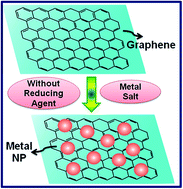
The integration of graphene with certain metallic nanoparticles, such as Au, Ag, Pt, Pd, Cu, etc., to produce a new generation of hybrid materials is a field of intense research nowadays. Graphene, being a single atom thick layer sheet of hexagonally arranged sp2 carbon atoms, has a prodigious number of free electrons, which can be used to reduce metallic ions to produce a hybrid material consisting of metal nanoparticles on the 2D fabric of graphene. Efforts were made to explore such property of the virgin graphene by careful in situ study using UV-visible (UV-vis) spectroscopy and transmission electron microscopy (TEM). The results indicate that it is possible to use surface potential of graphene to reduce Au3+, Ag+, Pt2+, Pd2+ and Cu2+ ions to prepare graphene–metal nanoparticle hybrids. The extensive TEM studies substantiate the finding of the formation of graphene decorated with metal nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...