Regio- and stereoselective synthesis of ensulfonamides/enamides via catalyst-free intermolecular addition of indoles/pyrroles/imidazole to allenamides†
Honghe Li,
Ting Ma,
Xiaoxiao Li* and
Zhigang Zhao*
College of Chemistry and Environmental Protection Engineering, Southwest University for Nationalities, Chengdu 610041, PR China. E-mail: lixiaoxiao.2005@163.com; zzg63129@163.com
First published on 29th September 2015
Abstract
A catalyst-free intermolecular addition of indoles, pyrroles, and imidazole to allenamides is reported. The reaction proceeds smoothly at 80 °C and provides a series of (E)-ensulfonamide derivatives in high yields with excellent regioselectivity. Two enamides 3k and 3l can also obtained when using acyl in place of tosyl as an amino-protecting group of allenamides.
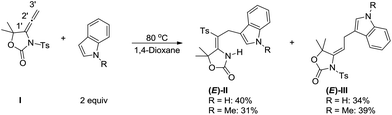
Sulfonamide functional groups have long been acclaimed as important structural motifs in drug discovery since the identification of a series of sulfonamide-containing drugs, such as sulfamethoxazole as an antibacterial agent, azosemide as a diuretic agent, sumatriptan as an antimigraine agent, and celecoxib as a COX-2 specific anti-inflammatory agent.1 The introduction of a sulfonamide group is often a useful practice in medicinal chemistry for improving pharmacological potency and/or the absorption, distribution, metabolism, and excretion (ADME) properties of the lead compound. Moreover, they are also important protected intermediates of primary and secondary amines with interesting biological activities.2 On the other hand, as the derivatives of sulfonamides, ensulfonamides are versatile building blocks that can be utilized in the heterocycle construction, asymmetric hydrogenation, and synthesis of bioactive molecules.3 Consequently, the interest in ensulfonamides has been long standing and the effective synthesis of these motifs, especially the stereocontrolled version, is still in urgent need. So far, ensulfonamides can be assembled by palladium(II)-catalyzed vinyl transfer from vinyl ethers to sulfonamides,4 Pd-catalyzed “Wacker-type” oxidative amidation of alkene,5 the functionalization of ynamides6 and rhodium(II)-mediated transformations of N-sulfonyl-1,2,3-triazoles.7 The functionalization of allenamides,8 which contain the desired sp2 carbon attached directly to the amide, stands out as an attractive alternative protocol for accessing ensulfonamides. Usually, the “electron-rich” π systems of allenamides are susceptible to electrophilic activation by transition metal9 or Brønsted acid10 providing zwitterionic intermediate, which can smoothly undergo condensation with a variety of nucleophilic agents. In contrast, they are robust to nucleophilic attack at the allene terminal carbons without catalyst. Yoshinao Tamaru and coworkers reported the first example that indole and methylindole served as a C-nucleophile proceeds nucleophilic addition at the allene terminal carbons of N-tosyl-4-vinylidene-2-oxazolidinone I at 80 °C under neutral conditions, but they provided a mixture of (E)-II and (E)-III in comparable amounts because of an intramolecular migration of the sulfonyl group from N to C2′ (Fig. 1).11
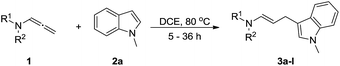
Our group have recently reported the gold catalyzed intermolecular [2 + 2] and [3 + 2] cycloaddition reaction of allenamides with olefins, azomethine imines and nitrones to provide multifunctional cyclobutanes, bipyrazolidin-1-one adducts and 4-methyleneisoxazolidine derivatives.12 In continuation of our program geared towards the functionalization of allenamides,13 we describe here a catalyst-free intermolecular addition of indoles, pyrroles, and imidazole to allenamides, providing (E)-ensulfonamides/enamides in high yields with excellent regioselectivity.
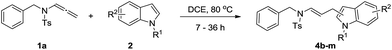
Our initial studies focused on the reaction of tosyl N-allenamide (1a) with N-methylindole (2a) for the optimization of reaction conditions (Table 1). Gratifyingly, the reaction outcome generally shows a regiochemical preference for the nucleophilic C3 attack by indole on the C3′ position of the allenamide providing (E)-ensulfonamide 3a as the only isomer in 54% yield together with minor amounts of C2-addition products of indole (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) at 120 °C in toluene after 25 h. Encouraged by these results, three other solvents, CHCl3, CH3CN, DCE were subsequently examined, and DCE showed the best reaction medium in terms of the yield (Table 1, entries 2–4). Variation of the number of equivalents of 2a from 3.0 to 2.0 lowed the conversion of 3a to 76% (Table 1, entry 5). The dramatically regio- and stereoselectivity was due to several reasons: firstly, the thermodynamic stability of the product. Secondly, C3 position of indole shows stronger nucleophilicity. Lastly, C2′ of the allenamide was to be nucleophilic centre because the electronic bias can be exerted through delocalization of the nitrogen lone pair of allenamide toward the allenic moiety. The structure and stereochemical identity of this adduct could be determined by the spectral analysis (JHC
1 ratio) at 120 °C in toluene after 25 h. Encouraged by these results, three other solvents, CHCl3, CH3CN, DCE were subsequently examined, and DCE showed the best reaction medium in terms of the yield (Table 1, entries 2–4). Variation of the number of equivalents of 2a from 3.0 to 2.0 lowed the conversion of 3a to 76% (Table 1, entry 5). The dramatically regio- and stereoselectivity was due to several reasons: firstly, the thermodynamic stability of the product. Secondly, C3 position of indole shows stronger nucleophilicity. Lastly, C2′ of the allenamide was to be nucleophilic centre because the electronic bias can be exerted through delocalization of the nitrogen lone pair of allenamide toward the allenic moiety. The structure and stereochemical identity of this adduct could be determined by the spectral analysis (JHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH > 12 Hz). Moreover, the structure of 3a can be corroborated through 3k (Table 2), which is consistent with literature.14
CH > 12 Hz). Moreover, the structure of 3a can be corroborated through 3k (Table 2), which is consistent with literature.14
| Entry | Allenamide, 1 | Product, 3 | Yieldc (%) |
|---|---|---|---|
| a Reagents and conditions: 1 (0.1 mmol), 2a (0.3 mmol) and DCE (anhydrous; 3 mL) at 80 °C.b In all cases, only one isomer was observed by NMR (see S5–S11).c Isolated yield. | |||
| 1 | 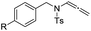 |
 |
|
| 2 | 1a, R = H | 3a | 92 |
| 3 | 1b, R = F | 3b | 95 |
| 4 | 1c, R = MeO | 3c | 72 |
| 5 | 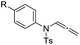 |
||
| 6 | 1d, R = H | 3d | 77 |
| 7 | 1e, R = Me | 3e | 82 |
| 8 | 1f, R = F | 3f | 92 |
| 9 | 1g, R = Br | 3g | 90 |
| 10 | 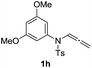 |
 |
62 |
| 11 | 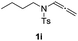 |
 |
96 |
| 12 | 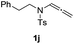 |
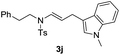 |
93 |
| 13 | 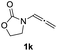 |
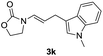 |
80 |
| 14 | 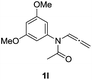 |
 |
50 |
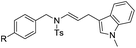
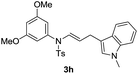
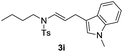
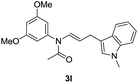
With the optimized reaction conditions in hand, we turned our attention to the scope of the intermolecular addition with respect to allenamides 1 and indoles 2 (Tables 2 and 3). As shown in Table 2, we were pleased to find that a series of allenamides with different substitution patterns worked very well in this reaction. 4-F- or 4-MeO-substituted benzyl allenamides were suitable for accessing ensulfonamides 3b and 3c in 95% and 72% yield in the presence of N-methylindole 2a. Phenyl allenamide 1d provided the desired ensulfonamide 3d in 77% yield. Subsequently, the substitution effect on the aryl ring of aryl allenamide was examined. While allenamides 1e, 1f and 1g bearing a 4-Me, 4-F or 4-Br group delivered 3e, 3f and 3g in high yields, 3,5-dimethoxy substituted aryl allenamide 1h gave the corresponding product 3h in moderate yield. When n-butyl allenamide 1i and phenylethyl allenamide 1j was utilized as the reactant, the addition products 3i and 3j were obtained in 96% and 93% yield, respectively. The reaction is also efficient with allenamides using acyl in place of tosyl as an amino-protecting group. Thus, 2-oxazolidinone allenamide 1k and 1l with an acyl substituent provided the corresponding adducts 3k and 3l in 80% yield and 50% yield respectively.
| Entry | Indole, 2 | Product, 4 | Yieldc (%) |
|---|---|---|---|
| a Reagents and conditions: 1a (0.1 mmol), 2 (0.3 mmol) and DCE (anhydrous; 3 mL) at 80 °C.b The ratio was determined by NMR (see S11–S13).c Isolated yield of the major product.d 1a (0.3 mmol)/2 (0.1 mmol). | |||
| 1 |  |
 |
|
| 2 | 2b, R2 = H | 4b (C3/C2 = 20/1) | 97 |
| 3 | 2c, R2 = 5-OMe | 4c (C3/C2 = 17/1) | 90 |
| 4 | 2d, R2 = 5-F | 4d (C3/C2 = 16/1) | 95 |
| 5d | 2e, R2 = 5-Br | 4e (C3/C2 = 19/1) | 61 |
| 6 | 2f, R2 = 4-Me | 4f (C3/C2 = 5/1) | 75 |
| 7 | 2g, R2 = 7-Me | 4g (C3/C2 = 7/1) | 70 |
| 8 |  |
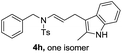 |
75 |
| 9 | 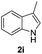 |
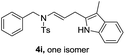 |
70 |
| 10 | 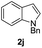 |
 |
71 |
| 11 | 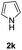 |
 |
82 |
| 12 | 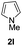 |
 |
80 |
| 13 | 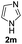 |
 |
81 |
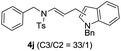
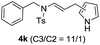
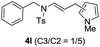
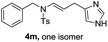
We next set out to explore the scope of indoles 2 in the presence of allenamide 1a (Table 3). The optimal conditions were found to be applicable to a wide range of indole derivants 2b–m and several substituents, such as Me, MeO, F, Br and benzyl on the indole ring were tolerated well. Indole 2b, 5-MeO or 5-F-substituted indole derivatives 2c–d were successfully converted into 4b–c in high yield which suggests that the electronic effect of substituents on the aromatic ring of indole does not affect the intermolecular addition of allenamides. But 5-bromoindole 2e gave the corresponding products 4e in moderate yield only when increasing the amount of 1a to 3 equivalent. 4-Methylindole and 7-methylindole provided the C3-addition products in good yield together with minor amounts of C2-adducts which were unseparated.15 Indoles bearing a 2-Me or N-benzyl were also viable to furnish 4h and 4j in 75% yield and 71% yield. Notably, 3-methylindole 2i could also provide the adduct 4i at the C2-position of indole in 70% yield due to the migration of the allyl group from C3 to C2 of indole. Interestingly, the catalyst-free intermolecular addition protocol was extended to pyrrole leading to C2-addition product 4k as the major isomer in 82% yield,15 while N-methylpyrrole provided C2-addition product 4l in 80% yield together with isolated C3-adduct in 15% yield.16 The regiochemical preference for the C2-position due to the more resonant type of the intermediate. Remarkably, imidazole is also an efficient nucleophile, which provided the C4-adduct 4m as the only isomer. As expected, the use of indole, pyrroles, and imidazole as nucleophile provided the desired (E)-ensulfonamides in good yield and high regioselectivity.
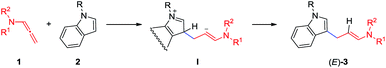
According to the report of Yoshinao Tamaru,11 we gave a plausible mechanism for this transformation in Scheme 1. The π*(C2′![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3′)–δ*(N–SO2) interaction makes the LUMO (C2′
C3′)–δ*(N–SO2) interaction makes the LUMO (C2′![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3′) low in energy and the double bond an excellent electron acceptor. Thus the nucleophilic addition of indoles providing (E)-3, in a sense, might be regarded as electrophilic aromatic substitution of 1; that is, despite the fact that 1 is a neutral species, it is even capable of undergoing the Friedel–Crafts type electrophilic aromatic substitution in the absence of any Lewis acid catalysts. Electrophilic addition of 1 to indoles and aromatization by deprotonation would provide (E)-3.
C3′) low in energy and the double bond an excellent electron acceptor. Thus the nucleophilic addition of indoles providing (E)-3, in a sense, might be regarded as electrophilic aromatic substitution of 1; that is, despite the fact that 1 is a neutral species, it is even capable of undergoing the Friedel–Crafts type electrophilic aromatic substitution in the absence of any Lewis acid catalysts. Electrophilic addition of 1 to indoles and aromatization by deprotonation would provide (E)-3.
Conclusions
In conclusion, we have developed a catalyst-free intermolecular addition of indole, pyrroles, and imidazole toward the distal double bond of allenamides. The reaction proceeds smoothly at 80 °C and provides a series of (E)-ensulfonamide/enamide derivatives in high yields with excellent regioselectivity. Further study of allenamides is currently ongoing in our laboratory.Acknowledgements
Financial support by the Fundamental Research Funds of Central Universities, Southwest University for Nationalities (2015NZYQN19) are gratefully acknowledged.Notes and references
- (a) A. Kalgutkar, R. Jones and A. Sawant, in Metabolism Pharmacokinetics and Toxicity of Functional Groups, ed. D. A. Smith, RSC, Cambridge, UK, 2010, ch. 5, RSC Drug Discovery Series No. 1 Search PubMed; (b) C. Hansch, P. G. Sammes and J. B. Taylor, Comprehensive Medicinal Chemistry, Pergamon Press, Oxford, UK, 1990, vol. 2, ch. 7.1 Search PubMed; (c) X. Chen, S. Hussain, S. Parveen, S. Zhang, Y. Yang and C. Zhu, Curr. Med. Chem., 2012, 19, 3578–3604 CrossRef CAS.
- P. G. M. Wuts and T. W. Greene, in Greene's Protective Groups in Organic Synthesis, John Wiley & Sons Inc, 4th edn, Hoboken, NJ, 2007, ch. 7 Search PubMed.
- (a) E. S. Tan, M. Miyakawa, J. R. Bunzow, D. K. Grandy and T. S. Scanlan, J. Med. Chem., 2007, 50, 2787–2798 CrossRef CAS PubMed; (b) E. S. Tan, E. S. Groban, M. P. Jacobson and T. S. Scanlan, Chem. Biol., 2008, 15, 343–353 CrossRef CAS PubMed; (c) E. S. Tan, J. C. Naylor, E. S. Groban, J. R. Bunzow, M. P. Jacobson, D. K. Grandy and T. S. Scanlan, ACS Chem. Biol., 2009, 4, 209–220 CrossRef CAS PubMed.
- J. L. Brice, J. E. Meerdink and S. S. Stahl, Org. Lett., 2004, 6, 1845–1848 CrossRef CAS PubMed.
- For the synthesis of ensulfonamides via oxidative amidation of alkene: (a) L. S. Hegedus and J. M. McKearin, J. Am. Chem. Soc., 1982, 104, 2444–2451 CrossRef CAS; (b) V. I. Timokhin, N. R. Anastasi and S. S. Stahl, J. Am. Chem. Soc., 2003, 125, 12996–12997 CrossRef CAS PubMed; (c) S. S. Stahl, Angew. Chem., Int. Ed., 2004, 43, 3400–3420 CrossRef CAS PubMed; (d) J. L. Brice, J. E. Harang, V. I. Timokhin, N. R. Anastasi and S. S. Stahl, J. Am. Chem. Soc., 2005, 127, 2868–2869 CrossRef CAS PubMed; (e) V. I. Timokhin and S. S. Stahl, J. Am. Chem. Soc., 2005, 127, 17888–17893 CrossRef CAS PubMed; (f) M. M. Rogers, V. Kotov, J. Chatwichien and S. S. Stahl, Org. Lett., 2007, 9, 4331–4334 CrossRef CAS PubMed; (g) V. Kotov, C. C. Scarborough and S. S. Stahl, Inorg. Chem., 2007, 46, 1910–1923 CrossRef CAS PubMed; (h) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318–5365 CrossRef CAS PubMed; (i) H. Hu, J. Tian, Y. Liu, Y. Liu, F. Shi, X. Wang, Y. Kan and C. Wang, J. Org. Chem., 2015, 80, 2842–2847 CrossRef CAS PubMed.
- For selected reports on the access of ensulfonamides via functionalization of ynamides: (a) G. Evano, A. Coste and K. Jouvin, Angew. Chem., Int. Ed., 2010, 49, 2840–2859 CrossRef CAS PubMed; (b) K. A. DeKorver, H. Li, A. G. Lohse, R. Hayashi, Z. Lu, Y. Zhang and R. P. Hsung, Chem. Rev., 2010, 110, 5064–5106 CrossRef CAS PubMed; (c) N. Saito, K. Saito, M. Shiro and Y. Sato, Org. Lett., 2011, 13, 2718–2721 CrossRef CAS PubMed; (d) Z. Lu, W. Kong, Z. Yuan, X. Zhao and G. Zhu, J. Org. Chem., 2011, 76, 8524–8529 CrossRef CAS PubMed; (e) Y. Kong, K. Jiang, J. Cao, L. Fu, L. Yu, G. Lai, Y. Cui, Z. Hu and G. Wang, Org. Lett., 2013, 15, 422–425 CrossRef CAS PubMed; (f) E. Rettenmeier, A. M. Schuster, M. Rudolph, F. Rominger, C. A. Gade and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2013, 52, 5880–5884 CrossRef CAS PubMed; (g) P. R. Walker, C. D. Campbell, A. Suleman, G. Carr and E. A. Anderson, Angew. Chem., Int. Ed., 2013, 52, 9139–9143 CrossRef CAS PubMed; (h) X.-N. Wang, H.-S. Yeom, L.-C. Fang, S. He, Z.-X. Ma, B. L. Kedrowski and R. P. Hsung, Acc. Chem. Res., 2014, 47, 560–578 CrossRef CAS PubMed; (i) Y. Yang, L. Wang, J. Zhang, Y. Jin and G. Zhu, Chem. Commun., 2014, 50, 2347–2349 RSC; (j) Y. Tokimizu, M. Wieteck, M. Rudolph, S. Oishi, N. Fujii, A. S. K. Hashmi and H. Ohno, Org. Lett., 2015, 17, 604–607 CrossRef CAS PubMed.
- For selected reports on the access of ensulfonamides via transformations of N-sulfonyl-1,2,3-triazoles; (a) N. Selander, B. T. Worrell, S. Chuprakov, S. Velaparthi and V. V. Fokin, J. Am. Chem. Soc., 2012, 134, 14670–14673 CrossRef CAS PubMed; (b) B. Chattopadhyay and V. Gevorgyan, Angew. Chem., Int. Ed., 2012, 51, 862–872 CrossRef CAS PubMed; (c) N. Selander, B. T. Worrell and V. V. Fokin, Angew. Chem., Int. Ed., 2012, 51, 13054–13057 CrossRef CAS PubMed; (d) T. Miura, T. Tanaka, K. Hiraga, S. G. Stewart and M. Murakami, J. Am. Chem. Soc., 2013, 135, 13652–13655 CrossRef CAS PubMed; (e) S. Chuprakov, B. T. Worrell, N. Selander, R. K. Sit and V. V. Fokin, J. Am. Chem. Soc., 2014, 136, 195–202 CrossRef CAS PubMed; (f) H. M. L. Davies and J. S. Alford, Chem. Soc. Rev., 2014, 43, 5151–5162 RSC; (g) D. J. Lee, J. Shin and E. J. Yoo, Chem. Commun., 2014, 50, 6620–6622 RSC; (h) P. Anbarasan, D. Yadagiri and S. Rajasekar, Synthesis-Stuttgart, 2014, 46, 3004–3023 CrossRef CAS; (i) S. Shin, Y. Park, C.-E. Kim, J.-Y. Son and P. H. Lee, J. Org. Chem., 2015, 80, 5859–5869 CrossRef CAS PubMed.
- (a) L. L. Wei, H. Xiong and R. P. Hsung, Acc. Chem. Res., 2003, 36, 773–782 CrossRef CAS PubMed; (b) P. E. Standen and M. C. Kimber, Curr. Opin. Drug Discovery Dev., 2010, 13, 645–657 CAS; (c) A. Deagostino, C. Prandi, S. Tabasso and P. Venturello, Molecules, 2010, 15, 2667–2685 CrossRef CAS PubMed; (d) T. Lu, Z. J. Lu, Z. X. Ma, Y. Zhang and R. P. Hsung, Chem. Rev., 2013, 113, 4862–4904 CrossRef CAS PubMed.
- For representative examples: (a) H. Faustino, F. Lopez, L. Castedo and J. L. Mascarenas, Chem. Sci., 2011, 2, 633–637 RSC; (b) S. Suarez-Pantiga, C. Hernandez-Diaz, M. Piedrafita, E. Rubio and J. M. Gonzalez, Adv. Synth. Catal., 2012, 354, 1651–1657 CrossRef CAS PubMed; (c) S. Suarez-Pantiga, C. Hernandez-Diaz, E. Rubio and J. M. Gonzalez, Angew. Chem., Int. Ed., 2012, 51, 11552–11555 CrossRef CAS PubMed; (d) J. Francos, F. Grande-Carmona, H. Faustino, J. Iglesias-Sigueenza, E. Diez, I. Alonso, R. Fernandez, J. M. Lassaletta, F. Lopez and J. L. Mascarenas, J. Am. Chem. Soc., 2012, 134, 14322–14325 CrossRef CAS PubMed; (e) Z.-X. Ma, S. He, W. Song and R. P. Hsung, Org. Lett., 2012, 14, 5736–5739 CrossRef CAS PubMed; (f) H. Faustino, I. Alonso, J. L. Mascarenas and F. Lopez, Angew. Chem., Int. Ed., 2013, 52, 6526–6530 CrossRef CAS PubMed; (g) S. Montserrat, H. Faustino, A. Lledos, J. L. Mascarenas, F. Lopez and G. Ujaque, Chem.–Eur. J., 2013, 19, 15248–15260 CrossRef CAS PubMed.
- (a) A. Navarro-Vazquez, D. Rodriguez, M. F. Martinez-Esperon, A. Garcia, C. Saa and D. Dominguez, Tetrahedron Lett., 2007, 48, 2741–2743 CrossRef CAS PubMed; (b) B. Guo, C. Fu and S. Ma, Chem. Commun., 2014, 50, 4445–4447 RSC; (c) C. Theunissen, B. Metayer, N. Henry, G. Compain, J. Marrot, A. Martin-Mingot, S. Thibaudeau and G. Evano, J. Am. Chem. Soc., 2014, 136, 12528–12531 CrossRef CAS PubMed; (d) C. Romano, M. Jia, M. Monari, E. Manoni and M. Bandini, Angew. Chem., Int. Ed., 2014, 53, 13854–13857 CrossRef CAS PubMed.
- M. Kimura, Y. Horino, M. Mori and Y. Tamaru, Chem.–Eur. J., 2007, 13, 9686–9702 CrossRef CAS PubMed.
- (a) X. X. Li, L. L. Zhu, W. Zhou and Z. L. Chen, Org. Lett., 2012, 14, 436–439 CrossRef CAS PubMed; (b) W. Zhou, X. X. Li, G. H. Li, Y. Wu and Z. L. Chen, Chem. Commun., 2013, 49, 3552–3554 RSC; (c) G. H. Li, W. Zhou, X. X. Li, Q. W. Bi, Z. Wang, Z. G. Zhao, W. X. Hu and Z. L. Chen, Chem. Commun., 2013, 49, 4770–4772 RSC.
- Z.-Q. Shen, X.-X. Li, J.-W. Shi, B.-L. Chen and Z. Chen, Tetrahedron Lett., 2015, 56, 4080–4083 CrossRef CAS PubMed.
- M. C. Kimber, Org. Lett., 2010, 12, 1128–1131 CrossRef CAS PubMed.
- The ratio of C3/C2 was determined by NMR, see the ESI†.
- The NMR spectra of C3-addition product of N-methylpyrrole, see the ESI†.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra10569b |
| This journal is © The Royal Society of Chemistry 2015 |