One-step fabrication of 3-methacryloxypropyltrimethoxysilane modified silica and investigation of fluorinated polyacrylate/silica nanocomposite films
Wei Yangab,
Liqun Zhua and
Yichi Chen*b
aSchool of Material Science and Engineering, Beihang University, Beijing 100191, China
bKey Laboratory of Bio-Inspired Smart Interfacial Science and Technology of Ministry of Education, Beijing Key Laboratory of Bio-Inspired Energy Materials and Devices, School of Chemistry and Environment, Beihang University, Beijing 100191, China. E-mail: chenych@buaa.edu.cn
First published on 24th June 2015
Abstract
Vinyl group containing silica (MSiO2) nanoparticles were prepared by the co-condensation of tetraethoxysilane (TEOS) with 3-methacryloxypropyltrimethoxysilane (MPS) and applied in the miniemulsion polymerization of methyl methacrylate (MMA), butyl acrylate (BA) and 1H,1H,2H,2H-heptadecafluorodecyl methacrylate (FA) to prepare fluorinated polyacrylate/silica nanocomposite particles. The morphology and particle size of MSiO2 was characterized by scanning electron microscopy (SEM). The nanocomposite films were investigated using atomic force microscopy (AFM), Fourier transform infrared (FT-IR) spectroscopy, water contact angle measurements, visible light spectroscopy, X-ray photoelectron spectroscopy (XPS) and thermogravimetry analyses (TGA). The effect of the amount of MSiO2 on the surface properties of the nanocomposite films was studied. Results showed that the MSiO2 nanoparticles presented a rough surface with a mean particle size of 200 nm. The nanocomposite film showed high transparency and the surface hydrophobicity of the fluorinated polyacrylate film was increased significantly by the incorporation of 0.04 g (0.46 wt% of monomers) of MSiO2 nanoparticles. The presence of MSiO2 contributed to the surface enrichment of the fluorinated components, and the nanocomposite film formation mechanism was proposed.
1. Introduction
Polymer/inorganic nanocomposite particles have gained much attention from scientists owing to the remarkable properties that can be obtained by the combination and structuration of the organic and inorganic components inside the nanoparticles.1–10 These nanocomposite materials are particularly promising in applications such as catalysis,11,12 surface coatings13–16 and biotechnologies.17 A number of approaches have been demonstrated for generating nanocomposite particles with different interesting morphologies, such as core–shell,18,19 raspberry-like,20–24 daisy-shaped,25 currant bun-like26 and snowman-like27 structures. A lot of nanocomposites have been prepared in the last decade via encapsulation or grafting of polymers onto the surface of inorganic components, of which silica is the most widely studied due to its great potential in commercial applications.28–31 The strategies for designing and fabricating polymer/silica nanocomposite particles have attracted wide interest because these composite particles exhibit enhanced mechanical and optical properties. Colloidal nanocomposite particles have various potential applications ranging from transparent, scratch-resistant coatings to durable exterior facade paints.32–35To improve the compatibility of silica with polymers, silica nanoparticles are necessarily pretreated with coupling agents,36–39 oleic acid40 or alcoholic components.41,42 However, as far as we know, there is no report focused on the one-step fabrication of MPS modified large silica nanoparticles (MSiO2). In addition, fluorinated polyacrylates have good adhesion to substrates, high transparency and amenable mechanical properties. They show many attractive characteristics including low surface energy, water and oil repellency, and high thermal, chemical, and weather resistance. Therefore, it is essential to spend effort on the preparation and investigation of the surface properties of fluorinated polyacrylate/silica nanocomposite films.
Herein, we describe the preparation of MPS modified silica nanoparticles by a one-step method of co-condensation of TEOS with MPS. Then, the fluorinated polyacrylate/silica (PFA/SiO2) nanocomposite particles were prepared from a miniemulsion polymerization. The microstructure of the particles and the properties of the nanocomposite film were investigated, moreover, a film formation mechanism of the nanocomposite film was proposed.
2. Experimental
2.1 Materials
Tetraethoxysilane (TEOS), methyl methacrylate (MMA) and butyl acrylate (BA) were purchased from Xilong Chemical Co., Ltd (Guangzhou, China). MMA and BA were purified by passing through a neutral alumina column to eliminate inhibitors before use. The 1H,1H,2H,2H-heptadecafluorodecyl methacrylate (FA) monomer was prepared in our lab. 3-Methacryloxypropyltrimethoxysilane (MPS), bought from Nanjing Xiangqian Chemical Co., Ltd, was analytical grade and used as received. Ammonium persulfate (APS) was obtained from Tianjin Bodi Chemical Co., Ltd and recrystalized before use. Sodium bicarbonate (NaHCO3), sodium dodecyl sulfate (SDS), hexdecane (HD) and ammonia (25 wt%) were all analytical grade and used without further purification. Deionized water was used for all polymerization and treatment processes.2.2 Methods
The morphology of MSiO2 was analyzed using a FESEM JSM-7500F instrument. The films formed on glass slides were sprayed with platinum (Pt) before observation. The morphology of the nanocomposite particles was characterized by transmission electron microscopy (TEM, JEM-2100, JEOL, Japan). A certain amount of latex was diluted with deionized water to a solid content of 1 wt%, then the diluted latex was dropped onto a carbon coated copper grid and negatively stained by a 2 wt% aqueous solution of phosphotungstic acid before TEM analysis. Fourier transform infrared (FT-IR) spectra were recorded on a NEXUS-470 FTIR analyzer (Nicolet, USA) from 4000 cm−1 to 500 cm−1 with a resolution of 8 cm−1. 19F NMR spectra were recorded using an AVANCE III 400 MHz Bruker spectrometer with CDCl3 as the solvent. Atomic force microscopy (Veeco DI, USA) was employed to investigate the film surface morphology in a tapping mode at ambient temperature by keeping a scan rate of 256 Hz and scan size of 5 μm × 5 μm. The transparency of the nanocomposite film was assessed by visible absorption spectrophotometry (UV-3600, SHIMADZU, Japan) from 400 nm to 800 nm, and the spectral slit width was set as 2 nm. The static contact angle of water on the latex films was measured using a sessile drop method with KRÜSS DSA20 (KRÜSS, Germany). 5 water contact angle values (5 μl per drop) on the film were averaged. X-ray photoelectron spectroscopy (XPS) studies were conducted on the dried nanocomposite film to get the chemical composition of the film surface in both survey and high-resolution mode on ESCALAB 250 systems equipped with an Al Kα X-ray source with a takeoff angle of 45°. Thermogravimetric analysis (TGA) was performed on a STA 3 Jupiter (Netzsch, Germany) from 50 °C to 500 °C at a heating rate of 10 °C min−1 with argon protection.2.3 One-step synthesis of MPS functionalized silica nanoparticles (MSiO2)
A novel method was suggested for silica modification in the process of silica formation, and the reaction mechanism between MPS and the silanol groups is depicted in Scheme 1. It is known that TEOS will undergo hydrolysis and condensation reaction processes in alkaline conditions. As shown in Scheme 1, there are a lot of silanol groups after the hydrolysis of TEOS, and the presence of a large amount of silanol groups makes the grafting of MPS with silica more efficient. Therefore, by appropriately adjusting the addition time of MPS, the silanol groups can react with MPS easily and gently at room temperature. Based on this theory, the following reaction was designed and implemented.Typically, 3.5 g of TEOS was mixed with 45 ml of ethanol, 10 ml of water and 2 ml of ammonia, and then stirred magnetically in a three-necked flask at room temperature. Subsequently, 0.3 g of MPS dissolved in 5 ml of ethanol was added dropwise into the flask. After completion of the addition, the reaction was continued for another 24 h at room temperature, and a white colored functionalized silica dispersion was obtained. The product was centrifuged and washed with ethanol three times, after which the silica was dried in an oven at 80 °C for 3 h, and then ground into powder before use.
2.4 Synthesis of the PFA/SiO2 nanocomposite particles
The PFA/SiO2 nanocomposite particles were prepared by miniemulsion polymerization. In a typical experiment, 0.04 g of MSiO2 (PFA4, the amount of MSiO2 was varied from 0 g to 0.15 g, and the nanocomposite was denoted as PFA0 to PFA15) was first dispersed in a mixture of monomers (4 g of MMA, 4 g of BA and 0.7 g of FA) and 0.4 g of HD with the aid of sonication for 20 min in an ice bath. Then, the dispersion was introduced into an aqueous solution of 0.08 g of SDS and 0.02 g of NaHCO3. After the mixture was sonicated for 20 min, the resultant miniemulsion was transferred into a 250 ml three-necked round-bottomed flask equipped with a mechanical stirrer, reflux condenser and nitrogen inlet. The polymerization was started with 0.08 g of APS at 70 °C and the reaction was complete after 6 h under a protective stream of nitrogen.3. Results and discussion
3.1 SEM and FT-IR analyses of MSiO2
The morphology and particle size of MSiO2 are shown in Fig. 1a. It is seen that the particles present a sphere morphology with a rough surface, from which small granules can be observed at the surface of MSiO2. As is well known, silica particles that are synthesized according to the Stöber procedure conventionally show a smooth surface because TEOS undergoes relatively complete hydrolysis and condensation reactions in ethanol/water solution. However, when MPS is added to the process of TEOS hydrolysis, MPS will also hydrolyze and further condense with the hydroxyl groups (–OH) of silanol (see Scheme 1), which results in silanol itself being unable to undergo complete condensation, therefore, silica particles showing a particular rough surface morphology were obtained. Fig. 1b presents the FT-IR spectra of pure silica and the MSiO2 synthesized in the experiment. As shown in Fig. 1b, after modification with MPS, a peak for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O appears in the MSiO2 spectrum. The peak at 1100 cm−1 is ascribed to the stretching vibration of Si–O–Si. The absorption at 950 cm−1 is assigned to Si–OH. The stretching vibration of Si–O occurs at 802 cm−1, and the peak at 470 cm−1 belongs to flexural vibration of Si–O. After modification, absorption of the carboxyl group (C
O appears in the MSiO2 spectrum. The peak at 1100 cm−1 is ascribed to the stretching vibration of Si–O–Si. The absorption at 950 cm−1 is assigned to Si–OH. The stretching vibration of Si–O occurs at 802 cm−1, and the peak at 470 cm−1 belongs to flexural vibration of Si–O. After modification, absorption of the carboxyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 1730 cm−1 occurred, which indicates that MPS was grafted onto the surface of the silica nanoparticles. The absorption of –OH occurs in both silica and MSiO2, indicating that there are still large numbers of –OH groups on the surface of MSiO2, although the surfaces of the nanoparticles were grafted with MPS molecules.
O) at 1730 cm−1 occurred, which indicates that MPS was grafted onto the surface of the silica nanoparticles. The absorption of –OH occurs in both silica and MSiO2, indicating that there are still large numbers of –OH groups on the surface of MSiO2, although the surfaces of the nanoparticles were grafted with MPS molecules.
3.2 19F NMR and FT-IR analyses
Fig. 2 presents the 19F NMR spectrum of PFA0. As shown in Fig. 2, the chemical shift at −81 ppm is assigned to –CF3, and the chemical shifts from −113 ppm to −127 ppm are ascribed to –CF2. Therefore, it is confirmed that FA has copolymerized with the acrylic monomers and that the PFA copolymer was obtained as expected.Fig. 3 presents the FT-IR spectra of the PFA/SiO2 nanocomposite films. The absorption peaks at 2950 cm−1 and 2870 cm−1 are due to the stretching vibrations of C–H. It can be seen that after incorporation of MSiO2 into fluorinated polyacrylate, the FT-IR spectrum of the film obviously changed. The peak at 3450 cm−1 is assigned to the stretching vibration of –OH, and the absorption at 1629 cm−1 belongs to the flexural vibration of –OH. It is seen that when MSiO2 was added into the polymerization, absorptions for –OH appear in the FT-IR spectrum. The characteristic absorption that appears at 806 cm−1 is due to the symmetric stretching vibration of Si–O. The characteristic peaks at 1240 cm−1 and 1170 cm−1 are the stretching vibration absorptions of C–F. Therefore, the results from the FT-IR spectra indicate that PFA/SiO2 nanocomposite particles were obtained as designed.
3.3 The surface properties of the PFA/SiO2 nanocomposite films
 | ||
| Fig. 7 XPS survey of the PFA4 and PFA8 films (a). Spectra for the PFA4 nanocomposite films: C1s scan (b), F1s scan (c), Si2p scan (d). | ||
| PFA0 | PFA4 | PFA8 | |
|---|---|---|---|
| C1s | 58.66 | 60.06 | 60.17 |
| O1s | 26.85 | 17.01 | 20.06 |
| F1s | 7.36 | 22.72 | 14.01 |
| Si2p | 0 | 0.22 | 1.97 |
| N1s | 2.79 | ||
| Na1s | 1.81 | ||
| S2p | 4.34 | 1.98 |
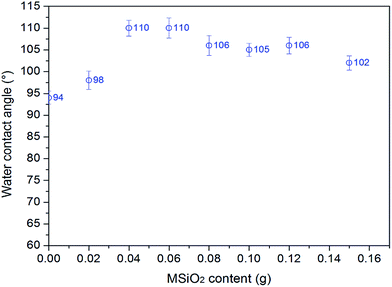
It can be seen that after the incorporation of MSiO2 into the PFA copolymer, the nanocomposite film surface is more fluorine enriched, and depending on what amount of MSiO2 is used in the miniemulsion polymerization, the silica content at the film surface is varied. When the MSiO2 content was increased from 0 g to 0.04 g, the surface hydrophobicity of the film increased, and with a further increase in the amount of MSiO2 in the polymerization, the film surface became more hydrophilic (see Fig. 5). According to XPS, this phenomenon can be explained as follows: a certain amount of MSiO2 (e.g. 0.04 g) makes the film more fluorine enriched and favors the film surface being more hydrophobic. As the amount of MSiO2 becomes more than 0.08 g, some aggregates of silica occur at the film surface, which causes a decrease in the film hydrophobicity. However, the nanocomposite films containing silica are more hydrophobic than PFA0 (no silica), which is due to the increased fluorinated segments and rough surface that were obtained simultaneously in the nanocomposite films.
In order to find the reason that causes the above results, the morphology of the nanocomposite particles was characterized and the result is shown in Fig. 8.
 | ||
| Fig. 8 The TEM image (a) and film-forming mechanism (b) of the PFA/SiO2 nanocomposite particles. The inset in (a) is the TEM image of MSiO2. | ||
As shown in Fig. 8a, the PFA polymeric particles show a white color in the TEM image because the nanocomposite particles were previously negatively stained by phosphotungstic acid, and all the polymeric particles exhibit a uniform sphere morphology. Meanwhile, it is observed that the MSiO2 particles showing high contrast (dark color) in the TEM image were encapsulated in the PFA polymeric particles. Due to the small amount of MSiO2 (0.46 wt% of the total monomers) in the miniemulsion, only a limited amount of MSiO2 can be found in the TEM image. However, this particular morphology of the nanocomposite particles is believed to affect film formation and the resulting film surface properties significantly. As a result, the probable film forming mechanism for the PFA/SiO2 nanocomposite particles is depicted in Fig. 8b. It is believed that the fluorinated polyacrylate particles are grafted onto silica due to the fact that vinyl groups are enriched at the MSiO2 surface (see Scheme 1), and every MSiO2 particle has several polymeric particles on its surface. When the hybrid latex is cast onto a glass substrate, the polymeric particles gradually form a polymer layer at the surface of MSiO2, and as the hybrid film is formed at last, more fluorinated segments will exist at the film surface than at that of the pure fluorinated polyacrylate. Therefore, this particular morphology of hybrid particles is useful for the surface enrichment of the fluorinated components at the surface after film formation.
| MSiO2 content (g) | ||||
|---|---|---|---|---|
| 0 | 0.02 | 0.08 | 0.15 | |
| T10 | 357.04 | 357.04 | 359.63 | 361.31 |
4. Conclusion
A novel process for the synthesis of MPS-functionalized large silica nanoparticles was described. Copolymerizing acrylic monomers of MMA and BA with 1H,1H,2H,2H-heptadecafluorodecyl methacrylate (FA) in the presence of MPS-functionalized silica leads to the formation of film-forming colloidal nanocomposite particles. Drying the aqueous nanocomposite dispersions results in highly transparent films, as judged by visible transmittance spectroscopy. The films showed improved surface hydrophobicity after the incorporation of a small amount of silica into the fluorinated polyacrylate, which is caused by the surface enrichment of the fluorinated components and increased surface roughness. This illustrates the utility of the in situ copolymerization approach for the synthesis of colloidal nanocomposite particles with excellent film-forming properties, good hydrophobicity and high transparency. Introducing only a small amount of silica into the fluorinated polyacrylate can promote the film hydrophobicity; this approach can also decrease the cost of the fluorinated component, while showing preferable surface properties, which will find potential applications in many fields.Acknowledgements
We are thankful for financial support from the National Natural Science Foundation of China (Grant No. 51173006).References
- T. Merkel, L. L. Hecht, A. Schoth, C. Wagner, R. Muñoz-Espí, K. Landfester and H. P. Schuchmann, Continuous Preparation of Polymer/Inorganic Composite Nanoparticles via Miniemulsion Polymerization, Springer International Publishing, 2015, pp. 345–370 Search PubMed.
- E. Bourgeat-Lami, F. D’Agosto and M. Lansalot, Synthesis of Nanocapsules and Polymer/Inorganic Nanoparticles Through Controlled Radical Polymerization At and Near Interfaces in Heterogeneous Media, Springer Berlin Heidelberg, 2015, pp. 1–39 Search PubMed.
- Y. Zhou, M. Cheng, X. Zhu, Y. Zhang, Q. An and F. Shi, J. Mater. Chem. A, 2013, 1, 11329 CAS.
- D.-H. Park, S.-J. Hwang, J.-M. Oh, J.-H. Yang and J.-H. Choy, Prog. Polym. Sci., 2013, 38, 1442–1486 CrossRef CAS PubMed.
- M. Naffakh, A. M. Díez-Pascual, C. Marco, G. J. Ellis and M. A. Gómez-Fatou, Prog. Polym. Sci., 2013, 38, 1163–1231 CrossRef CAS PubMed.
- R. Ladj, A. Bitar, M. M. Eissa, H. Fessi, Y. Mugnier, R. Le Dantec and A. Elaissari, Int. J. Pharm., 2013, 458, 230–241 CrossRef CAS PubMed.
- S. Kango, S. Kalia, A. Celli, J. Njuguna, Y. Habibi and R. Kumar, Prog. Polym. Sci., 2013, 38, 1232–1261 CrossRef CAS PubMed.
- Y. C. Huang, G. C. Welch, G. C. Bazan, M. L. Chabinyc and W. F. Su, Chem. Commun., 2012, 48, 7250–7252 RSC.
- Y. Du, S. Z. Shen, K. Cai and P. S. Casey, Prog. Polym. Sci., 2012, 37, 820–841 CrossRef CAS PubMed.
- J. Hu, M. Chen and L. Wu, Polym. Chem., 2011, 2, 760–772 RSC.
- R. J. Kalbasi and N. Mosaddegh, J. Solid State Chem., 2011, 184, 3095–3103 CrossRef CAS PubMed.
- M. Mari, B. Muller, K. Landfester and R. Munoz-Espi, ACS Appl. Mater. Interfaces, 2015, 7, 10727–10733 CAS.
- S. Bratskaya, A. Mironenko, R. Koivula, A. Synytska, A. Musyanovych, F. Simon, D. Marinin, M. Gobel, R. Harjula and V. Avramenko, ACS Appl. Mater. Interfaces, 2014, 6, 22387–22392 CAS.
- D. M. Bechi, M. A. d. Luca, M. Martinelli and S. Mitidieri, Prog. Org. Coat., 2013, 76, 736–742 CrossRef CAS PubMed.
- J. He, B. J. Chisholm, B. A. Mayo, H. Bao, J. Risan, D. A. Christianson and C. L. Rafferty, J. Coat. Technol. Res., 2012, 9, 423–431 CrossRef CAS.
- S. Bratskaya, A. Musyanovych, V. Zheleznov, A. Synytska, D. Marinin, F. Simon and V. Avramenko, ACS Appl. Mater. Interfaces, 2014, 6, 16769–16776 CAS.
- K. Rezwan, Q. Z. Chen, J. J. Blaker and A. R. Boccaccini, Biomaterials, 2006, 27, 3413–3431 CrossRef CAS PubMed.
- E. Bourgeat-Lami and J. Lang, J. Colloid Interface Sci., 1999, 210, 281–289 CrossRef CAS PubMed.
- X. Ding, J. Zhao, Y. Liu, H. Zhang and Z. Wang, Mater. Lett., 2004, 58, 3126–3130 CrossRef CAS PubMed.
- J. A. Balmer, A. Schmid and S. P. Armes, J. Mater. Chem., 2008, 18, 5722 RSC.
- J. A. Balmer, O. O. Mykhaylyk, J. P. A. Fairclough, A. J. Ryan, S. P. Armes, M. W. Murray, K. A. Murray and N. S. J. Williams, J. Am. Chem. Soc., 2010, 132, 2166–2168 CrossRef CAS PubMed.
- L. A. Fielding, J. Tonnar and S. P. Armes, Langmuir, 2011, 27, 11129–11144 CrossRef CAS PubMed.
- S. Reculusa, C. Poncet-Legrand, S. Ravaine, C. Mingotaud, E. Duguet and E. Bourgeat-Lami, Chem. Mater., 2002, 14, 2354–2359 CrossRef CAS.
- X. Cheng, M. Chen, S. Zhou and L. Wu, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 3807–3816 CrossRef CAS PubMed.
- S. Reculusa, C. Mingotaud, E. Bourgeat-Lami, E. Duguet and S. Ravaine, Nano Lett., 2004, 4, 1677–1682 CrossRef CAS.
- C. Barthet, A. J. Hickey, D. B. Cairns and S. P. Armes, Adv. Mater., 1999, 11, 408–410 CrossRef CAS.
- A. Perro, S. Reculusa, E. Bourgeat-Lami, E. Duguet and S. Ravaine, Colloids Surf., A, 2006, 284–285, 78–83 CrossRef PubMed.
- C.-H. Xue, Z.-D. Zhang, J. Zhang and S.-T. Jia, J. Mater. Chem. A, 2014, 2, 15001 CAS.
- Z. Wu, H. Wang, X. Tian, M. Xue, X. Ding, X. Ye and Z. Cui, Polymer, 2014, 55, 187–194 CrossRef CAS PubMed.
- X. Zhou, H. Shao and H. Liu, Colloid Polym. Sci., 2013, 291, 1181–1190 CAS.
- A. Schmid, P. Scherl, S. P. Armes, C. A. P. Leite and F. Galembeck, Macromolecules, 2009, 42, 3721–3728 CrossRef CAS.
- Z. Xue and H. Wiese, Int. Patent WO 03000760, 2003.
- J. Leuninger, F. Tiarks, H. Wiese and B. Schuler, Farbe Lack, 2004, 110, 30–38 CAS.
- Z. Xue and H. Wiese, US Pat. US7094830B2, 2006.
- C. Fu, X. Hu, Z. Yang, L. Shen and Z. Zheng, Prog. Org. Coat., 2015, 84, 18–27 CrossRef CAS PubMed.
- A. Krysztafkiewicz, T. Jesionowski and S. Binkowski, Colloids Surf., A, 2000, 173, 73–84 CrossRef CAS.
- T. Jesionowski and A. Krysztafkiewicz, Appl. Surf. Sci., 2001, 172, 18–32 CrossRef CAS.
- T. Jesionowski and A. Krysztafkiewicz, Colloids Surf., A, 2002, 207, 49–58 CrossRef CAS.
- C. Bartholome, E. Beyou, E. Bourgeat-Lami, P. Chaumont, F. Lefebvre and N. Zydowicz, Macromolecules, 2005, 38, 1099–1106 CrossRef CAS.
- X. Ding, Z. Wang, D. Han, Y. Zhang, Y. Shen, Z. Wang and L. Niu, Nanotechnology, 2006, 17, 4796–4801 CrossRef CAS.
- A. Schmid, S. P. Armes, C. A. Leite and F. Galembeck, Langmuir, 2009, 25, 2486–2494 CrossRef CAS PubMed.
- M. Fuji, S. Ueno, T. Takei, T. Watanabe and M. Chikazawa, Colloid Polym. Sci., 2000, 278, 30–36 CAS.
- M. J. Percy, J. I. Amalvy, C. Barthet, S. P. Armes, S. J. Greaves, J. F. Watts and H. Wiese, J. Mater. Chem., 2002, 12, 697–702 RSC.
- L. Yao, T. Yang and S. Cheng, J. Appl. Polym. Sci., 2010, 115, 3500–3507 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |