Synthesis and phase transfer of well-defined BiVO4 nanocrystals for photocatalytic water splitting†
Omer Yehezkeli*a,
Albert Harguindeya,
Dylan W. Domaillea,
Liangcan Hea and
Jennifer N. Cha*ab
aDepartment of Chemical and Biological Engineering, University of Colorado, Boulder, USA. E-mail: Jennifer.cha@colorado.edu; Omer.yehezkeli-1@colorado.edu
bMaterials Science and Engineering Program, University of Colorado, Boulder, USA
First published on 1st July 2015
Abstract
A method to synthesize BiVO4 nanoparticles and nanorods hydrothermally using sodium oleate as a capping ligand is presented. The BiVO4 nanocrystals possessed the expected blue shift in absorbance relative to bulk that occurs with scaling of particle size. Next, we transferred the BiVO4 nanoparticles from organic solvents to water using two different ligands. These particles were tested as water oxidation and dye reduction catalysts.
Extensive research has recently focused on discovering new solid state catalysts that can effectively split water using either externally biased1–5 or unbiased electrodes.6–17 While wide-band gap semiconductors like TiO2 (ref. 11) and WO3 (ref. 16, 18 and 19) have been studied as photocatalysts for water-splitting, more recent efforts have turned to BiVO4 due to its bandgap of 2.4 eV and ability to absorb in the visible.1,20 In the presence of electron scavengers such as AgNO3 or NaIO3, BiVO4 has also shown a strong ability to oxidize water and generate oxygen. Typically, BiVO4 has been grown by chemical vapor deposition (CVD)21,22 or chemical bath deposition (CBD)23 on conductive surfaces, which has produced roughly structured, high surface area photoactive electrodes. In other work, BiVO4 photocatalysts have been obtained by reacting potassium vanadate powder with Bi(NO3)3 to yield nanoclusters that showed 9% quantum efficiency (at 450 nm) as determined by the amount of oxygen generated under photoirradiation.24 Amorphous shaped BiVO4–Ru/SrTiO3:Rh (ref. 9) or BiVO4/graphene/SrTiO3 (ref. 7) composites have also been synthesized to run complete water splitting to oxygen and hydrogen under visible light. Furthermore, Li and co-workers recently used micron sized M/MnOx/BiVO4 and M/Co3O4/BiVO4 (M is a noble metal) composites to split water into oxygen and reduce methyl orange dye or NaIO3.25,26 The co-catalysts in this case were selectively deposited by photoreduction or oxidation of Pt or MnOx nanocrystals respectively on different facets of the BiVO4 structures, which led to better charge separation and lower back reactivities. More recently, co-catalysts like Co3O4, FeOOH and NiOOH were also electrodeposited on BiVO4 electrodes for improved water oxidation under illumination.27 In order to improve catalytic performance, various BiVO4 architectures have also been doped with low amounts (∼0.5–5%) of metals such as tungsten,28,29 molybdenum2 and phosphate30 which allow better hole transport and enhanced water oxidation abilities. Recently, published work by Yang and coworkers showed an elegent and straightforward method to assemble BiVO4 wires with Rh–SrTiO3 to achieve water splitting.31 Despite these successes in producing photoactive BiVO4 however, the synthesis of well-defined nanostructures of BiVO4 with control over both size and shape has remained challenging. Although a recent report showed successful hydrogen generation by nanosized BiVO4 in water presumably due to the change in energy levels as compared to bulk, the size and shape of the particles were less defined and the photocatalytic studies were run using surfactant coated particles which is known to impede electron and hole transfer.32 Other reports showed well defined BiVO4 NRs33 or tubes,34 but the sizes exceed 100 nm. Because of this, methods to obtain monodisperse and well-defined BiVO4 nanoparticles (NPs) and nanorods (NRs) were first pursued. As expected with particle scaling, a distinct blue shift in absorbance was observed from bulk indicating an increase in band gap energy. Next ligand exchange techniques that removed the large hydrocarbons from the particle surfaces and enabled their effective transfer to aqueous solutions were developed. Despite their blue shift in absorbance, when testing the photocatalytic performance of the BiVO4 NPs and NRs, a significant improvement in water oxidation as compared to micron-sized BiVO4 in the presence of sodium persulfate as an electron acceptor was observed. Finally, in the absence of any sacrificial donors it was found that the BiVO4 could reduce the dye molecule dichlorophenolindophenol (DCPIP) upon photoillumination in an oxygen-free environment.
In a typical synthesis, 1 g of Bi(NO3)3 was dissolved in 100 mL of DI water and stirred in a 250 mL reaction vessel for 10 min at room temperature. The temperature was next raised to 60 °C at which point 1.825 g of sodium oleate was added. The temperature was then raised to 80 °C followed by the dropwise addition of 250 mg of NH4VO3 dissolved in 50 mL of water. The reaction vessel was then sealed and the solution was continuously stirred during which the solution changed slowly from a milky white color to a yellow slurry. At different time points between 3 and 20 hours, the reactions were quenched by removing the flasks from heat and the products were collected by precipitation in water and centrifugation. The solids were then dissolved in toluene to obtain clear yellow-orange solutions, while the precipitated material (waste) was discarded. As shown in Fig. 1a, using these synthesis procedures we were able to obtain after 3 h ∼4.5 nm spherical nanoparticles that were well dispersed and stable in organic solvents (Fig. S1a†). X-ray diffraction (XRD) analyses of the as-synthesized nanoparticles showed that they possessed the tetragonal form of BiVO4 (Fig. S2a†). After 20 hours of synthesis we found that the spherical morphologies transitioned to nanorods (NRs) that were 4.7 nm in diameter and ∼20 nm in length (Fig. 1b, Fig. S1b†). The XRD measurement showed that like the NPs, the NRs also adopted the tetragonal form of BiVO4 a mixed form35,36 of tetragonal and monoclinic which could be attributed to the presence of other nanostructures (mainly NPs) in the NRs solutions, (Fig. S2b†). UV/vis absorbance measurements showed a distinct blue shift in the absorbance onset which increased as we moved from the NR samples to the NPs (Fig. S3†). Using the measured absorbance values, the band gaps of the NPs and NRs were determined to be 3.35 eV and 2.91 eV respectively. It should be noted that the NRs samples did contain a small portion of NPs. Furthermore by using different reaction times such as 6 or 12 h we obtained products that consisted of NPs largely with small amounts of NRs. We also examined the use of different pressure vessels for the reactions, which although led to the use of shorter reaction times to produce BiVO4 NRs, yielded primarily NR and NP mixtures.
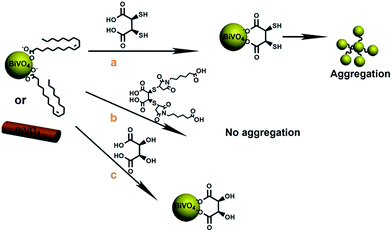
In order to test the photocatalytic activity of the as-synthesized BiVO4 NPs and NRs, methods to transfer the particles from toluene to water first needed to be developed. Although previous reports demonstrated the use of BiVO4 particles for water oxidation, these were typically run using either a suspension of surfactant coated BiVO4 nanoparticles or larger structures that were ligands free and therefore tended to precipitate out. After trying a number of small molecule ligands, we found we could achieve the best phase transfer of the as-synthesized BiVO4 NPs and NRs from toluene or chloroform to water with meso-2,3-dimercaptosuccinic acid (DMSA). For this, the as-synthesized oleic-capped BiVO4 NPs and NRs were first mixed with DMSA in toluene for 10 min followed by the addition of DI water and mixing. After stirring the solution for a few hours the BiVO4 NPs and NRs started to transfer to the aqueous phase (Fig. 2a). The DMSA molecule is thought to work through the carboxylic acid groups binding to the BiVO4 surface while leaving the thiols free to impart water solubility. Fig. 3 presents images of the ligand exchange process before, during and after the ligand exchange to the water phase. Greater evidence that the thiol groups of the DMSA are exposed from the NP surfaces was shown when the NPs started to aggregate a few hours after being transferred to the aqueous phase presumably due to thiol.
 | ||
| Fig. 3 The ligand exchange process (left), a mixture of the BiVO4 with DMSA in toluene (middle), after the addition of water (right), after mixing the two solutions overnight. | ||
Greater evidence that the thiol groups of the DMSA are exposed from the NP surfaces was shown when the NPs started to aggregate a few hours after being transferred to the aqueous phase presumably due to thiol oxidation (Fig. S4†). In order to circumvent this, we tried two different methods (i) react DMSA with maleimidocaproic acid (EMCA) prior to ligand exchange to prevent thiol oxidation (Fig. 2, route b) or (ii) react the NPs or NRs with tartaric acid (Fig. 2, route c). From these studies, we found that by either capping the free thiols on DMSA and simply replacing the thiols with alcohol groups could effectively prevent nanoparticle aggregation (Fig. S5†). The ligand exchange process was also observed to occur more quickly with the DMSA–EMCA molecules as compared to tartaric acid (TA) which required an overnight reaction for complete phase transfer.
It should be noted that although after several weeks slight aggregation was seen in both the DMSA–EMCA and TA conjugated nanoparticles, this was minute compared to the DMSA only treated samples (Fig. S6†). Furthermore, the capping ligands used were found to influence the optical properties of the particles where the DMSA–EMCA conjugated nanocrystals absorbed at longer wavelengths as compared to the TA bound BiVO4 (Fig. S7†).
After the successful phase transfer into water, the BiVO4 NPs and NRs were next tested for water oxidation. For this, sodium persulfate was added as a sacrificial electron acceptor and the samples were sealed and bubbled with argon to remove oxygen from the cuvette. The degassed samples were then placed under a solar simulator and the oxygen generated was monitored by a gas chromatogram (GC) equipped with a 5A column and TCD detector. Fig. 4a presents the oxygen produced from BiVO4 NPs and NRs capped with the TA or DMSA–EMCA. The largest amount of oxygen generated per particle was found to occur from the DMSA–EMCA conjugated NPs (blue triangles) with slightly less activity from the TA coated NPs (red squares). While the DMSA–EMCA conjugated NRs (green diamonds) showed comparable data to the TA bound NPs, much lower activities were seen from the TA coated NRs (black triangles). Overall the higher water oxidation activities observed with the NPs over the NRs can be attributed to the larger number of moles of NPs used as compared to NRs. Since the catalytic studies were performed per mass of BiVO4, it is expected that the smaller spherical NPs would yield a greater number of particles used than that for the larger NRs. In comparing however the nanoscale BiVO4 particles to micron sized BiVO4 synthesized using previously published procedures25 (Fig. S8†), significantly higher water oxidation was observed from the NPs and the DMSA-EMCA bound NRs. It must be noted here that this significant gain in catalysis was obtained despite the BiVO4 nanostructures absorbing primarily in the UV portion of the solar spectrum (Fig. S9†).
In addition to water oxidation, the photoreduction properties of the BiVO4 NPs and NRs were studied by using the dye molecule 2,6-dichlorophenol indophenol (DCPIP). For this, the particles were dispersed in water with DCPIP in a sealed cuvette, purged with nitrogen for 20 minutes to remove any free oxygen and then irradiated under 1 sun. In the absence of any sacrificial donors, the reduction of the dye was determined by measuring a decrease in absorbance at 525 nm. As shown in Fig. 4b, in the presence of the tartaric acid (TA) coated BiVO4 NPs, a distinct decrease in absorbance was observed and the solution became optically clear after 70 minutes (Fig. 4c). In order to verify that the dye was becoming reduced as opposed to being photodegraded, after photoillumination, sodium persulfate (Na2S2O8) was added as an oxidant to successfully regenerate the original state of the dye molecule (Fig. 4b, dashed line). Due to the larger band gap of the BiVO4 nanostructures, higher photoreduction activity was observed from the BiVO4 NPs (Fig. 4d, triangles) as compared to the micron-sized BiVO4 (Fig. 4d, circles). We also compared the effect of ligands on the BiVO4 nanocrystals on photoreduction as a function of particle morphology. As shown in Fig. 4e and f, while the TA conjugated NPs showed significantly better activity as compared to the DMSA–EMCA coated NPs, this phenomena was reversed with the BiVO4 NRs demonstrating that the ligands bound can have a direct effect on catalysis. While the effect of ligands on the nanoparticles was also seen with the water oxidation studies due to changes in nanocrystal absorption (Fig. 4a), the lowering in DCPIP photoreduction with the DMSA–EMCA coated NPs as compared with the TA conjugated NPs appeared counterintuitive. One possible explanation of this is that the combined use of DMSA and EMCA leads to an organic coating that possesses a much greater overall volume as compared to TA making it difficult for molecules such as DCPIP to reach the surface of the small spherical BiVO4 NPs. Therefore, while a small molecule like water may not have a difficult time to reach the small spherical NP surface, a much larger compound such as DCPIP might. In the case of the BiVO4 NRs however, since there is much less of an effect of surface crowding by any ligands attached, the observed widening in absorbance of the DMSA–EMCA coated NRs dominated to lead to an overall increase in photoreduction over that of with the DMSA–EMCA coated NRs was most likely due to the ligands causing a widening in the NR absorbance towards the visible that was not seen with the TA coated NRs (Fig. S7†). While it is possible that the negative charge of the acid groups on EMCA could also influence catalytic activity, as our experiments were run at pH 5, any charge effect should be minimized.
Conclusions
In conclusion, we have demonstrated here a new method to synthesize well-defined BiVO4 NPs and NRs that are monodisperse in size and shape. In addition, we showed for the first time two different strategies to transfer the as-synthesized BiVO4 particles from organic solvent to water through ligand exchange by using DMSA and EMCA or tartaric acid. These NPs and NRs were further tested for both water oxidation and dye reduction upon photoillumination in the absence of any sacrificial donors and in both cases, showed significantly higher catalytic activity as compared to micron-sized BiVO4 particles. These gains in activity were observed despite the BiVO4 nanocrystals absorbing primarily in the UV portion of the solar spectrum, illustrating the importance of having high surface area materials for catalysis. Lastly, we also showed that the ligands on the particles could have a direct influence on their optical properties and photocatalytic performance which was also dependent on nanocrystal size and morphology. Future studies will investigate the use of doping the BiVO4 nanostructures to shift the absorbance into the visible regime of the solar spectrum and thereby further increase their photocatalytic performance.Acknowledgements
The research was primarily supported by the U.S. Dept of Energy (DOE), Office of Science, Basic Energy Sciences (BES) under Award # DE-SC0006398 (synthesis, assembly, electrochemical measurements). In addition the research was supported by the Office of Naval Research under Award # N00014-09-01-0258 (microscopy characterization) and by the National Science Foundation under Award # DMR 1420736. O.Y. was supported by DOE DE-SC0006398.Notes and references
- Q. Jia, K. Iwashina and A. Kudo, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11564–11569 CrossRef CAS PubMed.
- J. A. Seabold, K. Zhu and N. R. Neale, Phys. Chem. Chem. Phys., 2013, 16, 1121–1131 RSC.
- M. W. Kanan and D. G. Nocera, Science, 2008, 321, 1072–1075 CrossRef CAS PubMed.
- R. Costi, E. R. Young, V. Bulović and D. G. Nocera, ACS Appl. Mater. Interfaces, 2013, 5, 2364–2367 CAS.
- O. Yehezkeli, D. R. B. de Oliveira and J. N. Cha, Small, 2014, 6, 667 Search PubMed.
- K. Maeda and K. Domen, Angew. Chem., Int. Ed., 2012, 51, 9865–9869 CrossRef CAS PubMed.
- A. Iwase, Y. H. Ng, Y. Ishiguro, A. Kudo and R. Amal, J. Am. Chem. Soc., 2011, 133, 11054–11057 CrossRef CAS PubMed.
- F. F. Abdi, L. Han, A. H. M. Smets, M. Zeman, B. Dam and R. van de Krol, Nat. Commun., 2013, 4, 2195 Search PubMed.
- Q. Jia, A. Iwase and A. Kudo, Chem. Sci., 2014, 5, 1513–1519 RSC.
- K. Kalyanasundaram, E. Borgarello and M. Grátzel, Helv. Chim. Acta, 1981, 64, 362–366 CrossRef CAS PubMed.
- S. U. M. Khan, M. Al-Shahry and W. B. Ingler, Science, 2002, 297, 2243–2245 CrossRef CAS PubMed.
- L. Liao, Q. Zhang, Z. Su, Z. Zhao, Y. Wang, Y. Li, X. Lu, D. Wei, G. Feng, Q. Yu, X. Cai, J. Zhao, Z. Ren, H. Fang, F. Robles-Hernandez, S. Baldelli and J. Bao, Nat. Nanotechnol., 2014, 9, 69–73 CrossRef CAS PubMed.
- G. Liu, J. Shi, F. Zhang, Z. Chen, J. Han, C. Ding, S. Chen, Z. Wang, H. Han and C. Li, Angew. Chem., Int. Ed., 2014, 53, 7295–7299 CrossRef CAS PubMed.
- K. Maeda, ACS Catal., 2013, 3, 1486–1503 CrossRef CAS.
- K. Maeda, K. Teramura, T. Takata, M. Hara, N. Saito, K. Toda, Y. Inoue, H. Kobayashi and K. Domen, J. Phys. Chem. B, 2005, 109, 20504–20510 CrossRef CAS PubMed.
- K. Maeda, A. Xiong, T. Yoshinaga, T. Ikeda, N. Sakamoto, T. Hisatomi, M. Takashima, D. Lu, M. Kanehara, T. Setoyama, T. Teranishi and K. Domen, Angew. Chem., Int. Ed., 2010, 49, 4096–4099 CrossRef CAS PubMed.
- F. Zhang, A. Yamakata, K. Maeda, Y. Moriya, T. Takata, J. Kubota, K. Teshima, S. Oishi and K. Domen, J. Am. Chem. Soc., 2012, 134, 8348–8351 CrossRef CAS PubMed.
- R. Abe, T. Takata, H. Sugihara and K. Domen, Chem. Commun., 2005, 3829–3831 RSC.
- J. Su, X. Feng, J. D. Sloppy, L. Guo and C. A. Grimes, Nano Lett., 2011, 11, 203–208 CrossRef CAS PubMed.
- W. Luo, Z. Yang, Z. Li, J. Zhang, J. Liu, Z. Zhao, Z. Wang, S. Yan, T. Yu and Z. Zou, Energy Environ. Sci., 2011, 4, 4046–4051 CAS.
- E. Alarcón-Lladó, L. Chen, M. Hettick, N. Mashouf, Y. Lin, A. Javey and J. W. Ager, Phys. Chem. Chem. Phys., 2013, 16, 1651–1657 RSC.
- W. Luo, Z. Wang, L. Wan, Z. Li, T. Yu and Z. Zou, J. Phys. D: Appl. Phys., 2010, 43, 405402 CrossRef.
- J. Su, L. Guo, N. Bao and C. A. Grimes, Nano Lett., 2011, 11, 1928–1933 CrossRef CAS PubMed.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459–11467 CrossRef CAS.
- R. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Nat. Commun., 2013, 4, 1432 CrossRef PubMed.
- R. Li, H. Han, F. Zhang, D. Wang and C. Li, Energy Environ. Sci., 2014, 7, 1369–1376 CAS.
- T. W. Kim and K.-S. Choi, Science, 2014, 343, 990–994 CrossRef CAS PubMed.
- H. S. Park, H. C. Lee, K. C. Leonard, G. Liu and A. J. Bard, ChemPhysChem, 2013, 14, 2277–2287 CrossRef CAS PubMed.
- D. K. Zhong, S. Choi and D. R. Gamelin, J. Am. Chem. Soc., 2011, 133, 18370–18377 CrossRef CAS PubMed.
- W. J. Jo, J.-W. Jang, K. Kong, H. J. Kang, J. Y. Kim, H. Jun, K. P. S. Parmar and J. S. Lee, Angew. Chem., Int. Ed., 2012, 51, 3147–3151 CrossRef CAS PubMed.
- B. Liu, C.-H. Wu, J. Miao and P. Yang, ACS Nano, 2014, 8, 11739 CrossRef CAS PubMed.
- S. Sun, W. Wang, D. Li, L. Zhang and D. Jiang, ACS Catal., 2014, 4, 3498–3503 CrossRef CAS.
- Y. Zhang, Y. Guo, H. Duan, H. Li, C. Sun and H. Liu, Phys. Chem. Chem. Phys., 2014, 16, 24519–24526 RSC.
- Y. Sun, Y. Xie, C. Wu, S. Zhang and S. Jiang, Nano Res., 2010, 3, 620–631 CrossRef CAS PubMed.
- D. Ke, T. Peng, L. Ma, P. Cai and K. Dai, Inorg. Chem., 2009, 48, 4685–4691 CrossRef CAS PubMed.
- H. Fan, T. Jiang, H. Li, D. Wang, L. Wang, J. Zhai, D. He, P. Wang and T. Xie, J. Phys. Chem. C, 2012, 116, 2425–2430 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD, UV/vis, TEM. See DOI: 10.1039/c5ra10454h |
| This journal is © The Royal Society of Chemistry 2015 |