Nanoengineered drug-releasing aluminium wire implants: comparative investigation of nanopore geometry, drug release and osteoblast cell adhesion
Abstract
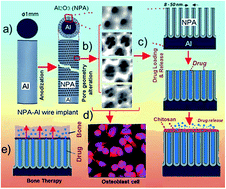
In this study, drug-releasing aluminium (Al) wire implants featuring nanoporous alumina (NPA) layers produced by different anodization approaches are systematically investigated as potential platforms for localized drug delivery and bone therapy. NPA-Al wires are fabricated by symmetric and asymmetric two-step anodization approaches in sulphuric and oxalic acid electrolytes. The top surface of the resulting NPA layers displays different geometric features and a nanoporous structure. While a symmetric two-step anodization process yields nanoporous layers based on single nanopore cells, asymmetric anodization leads to a hierarchical nanoporous structure composed of 2–10 nanopores per cell, which can be precisely engineered by the anodization conditions. The drug-releasing performance of the resulting NPA layers is assessed through a series of in vitro studies. The results reveal that NPA-Al wire implants with hierarchical nanoporous structures present enhanced drug loading and release capabilities as compared to implants based on single nanopore cells. Biopolymer (chitosan) coating layers were incorporated onto these NPA-Al drug-loaded wire implants in order to control the release of the drug over a longer time period. Finally, the potential osseointegration of NPA-Al implants is evaluated by osteoblast cell adhesion experiments. NPA-Al implants with a hierarchical nanopore structure show significantly greater osteoblast cell attachment as compared to NPA-Al wires produced by symmetric anodization and their chitosan coated forms. Overall, this study demonstrates that drug releasing NPA-Al wire implants with precisely engineered nanopore structures have great potential as implant platforms for treatment of localized diseases such as bone cancer and osteomyelitis.

 Please wait while we load your content...
Please wait while we load your content...