Copper catalyzed synthesis of unsymmetrical diaryl sulfones from an arenediazonium salt and sodium p-toluenesulfinate†
Abstract
Aryl sulfones have been for the first time synthesized by the reaction of sodium p-toluenesulfinate and arenediazonium salts using a CuI catalyzed homogeneous system. The developed protocol is a simple and efficient new route for the synthesis of diaryl sulfones with excellent product yields. The mild reaction conditions tolerate a range of functional groups. The best results were obtained with CuI, N,N′-dimethylethylenediamine, TBAI and K2CO3 in dimethyl sulfoxide at 100 °C under an inert atmosphere.
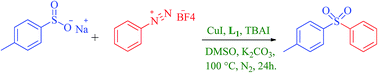

 Please wait while we load your content...
Please wait while we load your content...