Facile fabrication of nanostructured NiCo2O4 supported on Ni foam for high performance electrochemical energy storage
Changhui Wangab,
Xiong Zhanga,
Chen Liab,
Xianzhong Suna and
Yanwei Ma*a
aInstitute of Electrical Engineering, Chinese Academy of Sciences, Beijing 100190, China. E-mail: ywma@mail.iee.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 10th September 2015
Abstract
Intensive research in the area of electrochemical energy storage (EES) in the past decade has been inspired by the demand for EES in handheld electronic devices, transportation, and storage of renewable energy for the power grid. It has become necessary to find novel electrode materials with high performance, low cost and advanced electrode architecture to meet the large-scale commercial applications of EES. We developed a facile, green and energy-saving technique to fabricate cost-effective nanostructured NiCo2O4 supported on Ni foam (NiCo2O4/Ni foam) with a unique microstructure. As a binder-free electrode material, a high specific capacitance of 760 F g−1 was achieved with excellent cyclability at a current density of 1 A g−1. In conjunction with the superior electrode architecture, the nanoplate-like NiCo2O4/Ni foam with optimal performance has the potential to meet the needs of its wide commercial application for high-efficiency EES. The preparation method may be extended to other metal oxide/hydroxide-based materials with outstanding nanostructures for electronic, magnetic, optical, photochemical, and catalytic applications.
1. Introduction
Intensive research in the area of electrochemical energy storage (EES) in the past decade has been inspired by the demand for EES in handheld electronic devices, transportation, and the storage of renewable energy for the power grid.1,2 However, the commercial applications of EES are still limited due to low performance, high cost or various difficulties in the large scale-up fabrication of the electrode materials. Therefore, it has become necessary to find novel electrode materials with high performance, low cost and advanced electrode architecture.3,4NiCo2O4 has been suggested as a promising cost-effective and scalable alternative since it offers many advantages such as low cost and high theoretical capacitance. The preparation techniques for it include coprecipitation,5 combustion,6 hydrothermal methods,7 thermal decomposition,8 sol–gel processing,9 etc. Moreover, in general, the active material needs to be mixed with a conductive agent and a binder to make a paste and applied to current collectors for electrochemical evaluation.10,11 Apparently, the complex fabrication process makes it much more difficult to boost the performance and scale-up of the active electrode materials. An emerging advanced technique is to grow electroactive nanostructured materials on conductive substrates to be directly used as binder-free electrodes for EES, such as single-crystalline NiCo2O4 nanoneedle arrays, NiCo2O4 nanowire arrays, or ultrathin mesoporous NiCo2O4 nanosheets supported on Ni foam or Ti foil,12–14 by a one-step hydrothermal method,12 a multi-step synthetic process including potentiostatic deposition,13 or chemical bath deposition (CBD)14 followed by thermal transformation.13,14 The electrode design avoids the tedious procedure of mixing and coating of the slurry, and makes the active electrode materials free of auxiliary components like conductive agents and binders besides plenty of “dead surface”. While the NiCo2O4-based electrode materials obtained via the above routes have displayed high performance, there has been a delay in the commercial application of NiCo2O4 materials in EES, because their synthetic methodologies suffer high energy costs, an elevated temperature, complex equipment, complicated techniques, environmental pollution, low yield, etc.
In this work, we report a facile, green approach to grow nanostructured NiCo2O4 on Ni foam (NiCo2O4/Ni foam) with an optimal microstructure as a binder-free electrode material for EES. The as-fabricated nanoplatelet-like NiCo2O4/Ni foam possessed a high specific capacitance of 760 F g−1 at a current density of 1 A g−1 and an excellent cyclability, remaining at over 96.3% after 1000 cycles, at a high load mass of 1.46 mg cm−2. Benefitting from the superior electrode architecture, the high performance nanostructured NiCo2O4/Ni foam has the potential to meet the needs of its wide commercial application for high-efficiency EES.
2. Experimental
2.1. Materials
Ni(NO3)2·6H2O (≥98.0), Co(NO3)2·6H2O (≥99.0), NaOH (≥96.0%) and ethylene glycol (EG, ≥96.0%) were used as source materials without further purification. Nickel foams (purity: >99.5%) were cleaned ultrasonically in a 37 wt% HCl solution for 5 min and washed with deionized water and ethanol, before being dried in an oven at 60 °C.15 All of the reagents were analytical grade.2.2. Preparation of the nanostructured NiCo2O4/Ni foam
In our synthesis process, two steps are involved: (1) preparation of the precursor ethylene glycol intercalated cobalt and nickel layered double hydroxide nanosheets on Ni foam (E-Co–Ni LDH/Ni foam) via a facile modified coprecipitation method16 followed by an effective vacuum freeze-drying technique, and (2) preparation of the end product, nanoplatelet-like NiCo2O4/Ni foam, by a simple thermal transformation route. Typically, specific amounts of Co(NO3)2·6H2O (8 mmol) and Ni(NO3)2·6H2O (4 mmol) were dissolved in 80 mL of deionized water with 20 mL of EG. The claret solution above was then added dropwise to colorless EG containing 26 mmol of NaOH under constant high-speed stirring at room temperature. After undergoing a process of Ostwald ripening, centrifuging, and washing thoroughly, the resulting reaction product was redispersed in a mixed solution consisting of 135 mL of EG and 40 mL of deionized water by an ultrasonic vibration process to form a uniform and stable suspension of E-Co–Ni LDH nanosheets. Subsequently, the bottom part (1 cm × 1 cm in square shape) of a piece of clean nickel foam (3.5 cm × 1 cm in rectangular shape) was immersed in the dispersion for several seconds, followed by pre-freezing treatment in liquid nitrogen and subsequent vacuum freeze-drying at −60 °C under a vacuum of less than 10 Pa overnight. After that, the precursor E-Co–Ni LDH/Ni foam was obtained. Next, the precursor was placed in a furnace to be annealed at a relatively low temperature of 260 °C in air for 120 min at a slow heating of 1 °C min−1, and the final nanostructured NiCo2O4/Ni foam was achieved.2.3. Characterization
X-ray diffraction (XRD) patterns of the samples were recorded with an X-ray powder diffractometer (CuKα, Bruker D8). Thermogravimetric analysis and differential scanning calorimetry analysis (TG-DSC) of the powder samples were measured using a Netzsch STA 449C instrument with a heating rate of 10 °C min−1 in air. The morphology of the products was characterized with a field emission scanning electron microscope (FESEM, HITACHI S-4800) and an analytical transmission electron microscope (TEM, JEOL JEM2010) operated at 200 kV.2.4. Electrochemical measurement
Chronopotentiometry and cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) were conducted using a three-electrode mode in a 1 M NaOH solution using a CHI 660C electrochemical workstation. The working electrode was the nanostructured NiCo2O4/Ni foam used as a binder-free electrode. The loading level of the active material was controlled to be at least 1 mg cm−2. The reference electrode and counter electrode were a saturated calomel electrode (SCE) and platinum foil, respectively.3. Results and discussion
As shown in Fig. 1a, the peaks in the XRD pattern (c) of the precursor can be indexed to the (003), (006), (101) and (110) plane reflections of both α-Co(OH)2 and α-Ni(OH)2 LDHs,16,17 except for the three typical peaks originating from the Ni substrate.13 A low angle reflection appears at 9.64 Å (9.17°) followed by another reflection at about one-half this spacing, 4.78 Å (18.95°). The interlayer distance (d003’s) of ca. 9.64 Å is enlarged, compared to that (ca. 7.9 Å) reported in the literature.18 Together with the coherent shift of the (00l) peaks, i.e. the (003) and (006) plane reflections, as a whole, it can be confirmed that the precursor of EG intercalated and chemically bonded Co–Ni hydroxides was formed on the Ni foam.19–21 | ||
| Fig. 1 XRD patterns (a) of the precursor and the end product; TG-DSC curves (b) of the precursor powder. | ||
From the TG-DSC of the precursor powder in Fig. 1b, we can attribute the abrupt weight loss at 255–290 °C to the combustion of the intercalated EG and the decomposition of Co–Ni hydroxide into the relative oxides, while the mass loss above 290 °C can be attributed to the thermal decomposition of E-Co–Ni hydroxide and the corresponding metal oxides.22–24 When the precursor E-Co–Ni LDH/Ni foam is annealed at a relatively low temperature of 260 °C, the formation of the nanostructured NiCo2O4 will be accompanied by the evolution of much more gas including H2O (g) and CO2 (g) from the combustion of EG. This may favor the generation of a porous structure, which is good for loading and penetration of the electrolyte, charge transport and ion diffusion in electrochemical reactions.4 As exhibited in the XRD pattern (b) of the end product in Fig. 1a, the weak but distinctive NiCo2O4 reflections can be seen coming from the (111), (311), (511), (110) reflection planes.6,16 The broad diffraction peaks indicate that nanosized NiCo2O4 was achieved.
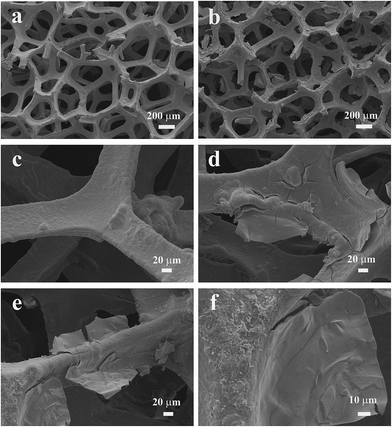
The FESEM images of the samples are shown in Fig. 2a–f. For the nanostructured NiCo2O4/Ni foam, the 3D grid structure with hierarchical macro-porosity of the Ni foam is well retained (Fig. 2b) and the surface is rougher than that of the pristine Ni foam (Fig. 2a). Moreover, uniform coverage of the nanostructured NiCo2O4 can be seen on the whole of the Ni foam substrate (Fig. 2d). And one can recognize that microplate-like NiCo2O4 has grown on the Ni foam substrate (Fig. 2e and f).
TEM measurements were performed to investigate the inherent structural and morphological characteristics of the products (Fig. 3a–c). A nanoplatelet-like shape of the nanostructured NiCo2O4 can be recognized (Fig. 3a), which possesses a narrow size distribution of 2–10 nm. The nanosized NiCo2O4 material provides a high specific surface area, and short electron and ion transport pathways to enhance the capacitive performance.4,12 A large number of mesopores and micropores can be observed in Fig. 3b and c, coming from the aggregates of the nanoplatelet-like particles (Fig. 3b) and the defects in the nanoparticles (Fig. 3c), respectively. Additionally, the diffused spotty SAED pattern with well-defined rings (inset of Fig. 3b) indicates the polycrystalline characteristics.
 | ||
| Fig. 3 TEM images of NiCo2O4/Ni foam (a and c), and the TEM image of NiCo2O4/Ni foam (b) with corresponding SAED pattern in the inset. | ||
To further evaluate the merits of the optimal inherent microstructure and the unique architecture, we directly applied the nanoplatelet-like NiCo2O4/Ni foam as an electrode for EES. Fig. 4a shows the CV curves at various scan rates ranging from 5 to 100 mV s−1 within a potential window of −0.15 to 0.5 V. Clearly, a pair of well-defined redox peaks is visible owing to the faradaic capacitive behavior. Their shapes have not significantly changed with the increase of the scan rate, revealing the ideal capacitive behavior of the nanoplatelet-like NiCo2O4/Ni foam.12 There are battery-like voltage plateaus in the galvanic charge–discharge (CD) curves (Fig. 4b), which match well with the redox peaks in the CV curves. The corresponding specific capacitance values can be calculated by the following relationship:25
| Cm = I × Δt/ΔV × m | (1) |
As expected, the nanoplatelet-like NiCo2O4/Ni foam shows a high specific capacitance of 760 F g−1 at a current density of 1 A g−1 and excellent rate capacity (Fig. 4a, b and e). The electrochemical stability of the sample was examined by repeating the CV cycles at 10 mV s−1. Interestingly, the specific capacitance increases before 500 cycles of fully electrochemical activation (Fig. 4c and d). The specific capacitance retention ratio reaches over 96.3% (732.2 F g−1) after 1000 cycles based on the maximum value, indicating the unique structural stability of the sample (Fig. 4f).
As discussed before, the advantageous intrinsic structure of the nanoplatelet-like NiCo2O4/Ni foam plays an important role in the high electrochemical performance. The high specific surface affords a high density of active sites to increase the redox reaction.9,26 Large quantities of micro/mesopores make the mass transfer of electrolyte easier, and the interior hollow spaces may accommodate the volume change in the redox reaction and enhance the cyclability of the electrode material as a result.9,27,28 Simultaneously, the hybrid architecture structure is undoubtedly indispensable to the outstanding electrochemical performance, since it provides a more favourable and quicker pathway for ions to penetrate owing to the direct loading of the active material on the current collector. As shown in Fig. 5, the Nyquist plot of the three-electrode system with the NiCo2O4/Ni foam electrode shows a straight line in the low-frequency region and an inconspicuous arc in the high frequency region, at an applied potential of 0.006 V (vs. SCE). The magnitude of the equivalent series resistance (ESR) (0.6 Ω) is obtained from the x-intercept of the plot for the NiCo2O4/Ni foam electrode material. The inconspicuous arc in the high frequency region indicates that the electronic resistance is low between the nanoplatelet-like NiCo2O4/Ni foam. The portion of the curve with a slope of 45°, called the Warburg resistance, comes from the frequency dependence of ion diffusion in the electrolyte to the electrode interface. The short Warburg curve in the plot suggests that the sample has a short diffusion path, which may facilitate the efficient access of electrolyte ions to the active material surface.29 Together with the facile, relatively low temperature fabrication process, the high performance nanoplatelet-like NiCo2O4/Ni foam is promising as a candidate electrode material for wide practical application for EES.
 | ||
| Fig. 5 Nyquist plots for the NiCo2O4/Ni foam electrode material at an applied potential of 0.006 V (vs. SCE). Z′: real impedance. Z′′: imaginary impedance. Inset shows an enlarged scale. | ||
4. Conclusions
In summary, we developed a facile, green, and energy-saving preparation technique to achieve cost-effective nanostructured NiCo2O4/Ni foam for EES. The nanoplatelet-like particles of 2–10 nm provide a high density of active sites and an advantageous porous structure for electrochemical reactions. As a binder-free electrode material, a high specific capacitance of 760 F g−1 was obtained at 1 A g−1 with excellent structural stability, remaining at over 96.3% after 1000 cycles. The study may facilitate the successful commercial application of nanoplatelet-like NiCo2O4/Ni foam for high-efficiency EES. The preparation procedure may be extended to other metal oxide/hydroxide-based material systems for electronic, magnetic, optical, photochemical, and catalytic applications, and further relevant work will follow in our future research.Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (Grant 51472238).References
- Y. Gogotsi and P. Simon, Science, 2011, 334, 917–918 CrossRef CAS PubMed.
- H. Y. Chen, F. Cai, Y. R. Kang, S. Zeng, M. H. Chen and Q. W. Li, ACS Appl. Mater. Interfaces, 2014, 6, 19630–19637 CAS.
- J. Jiang, Y. Y. Li, J. P. Liu, X. T. Huang, C. Z. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166–5180 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- Y. Kobayashi, X. Ke, H. Hata, P. Schiffer and T. E. Mallouk, Chem. Mater., 2008, 20, 2374–2381 CrossRef CAS.
- S. Verma, H. M. Joshi and T. Jagadale, J. Phys. Chem. C, 2008, 112, 15106–15112 CAS.
- Y. NuLi, P. Zhang, Z. P. Guo, H. K. Liu and J. Yang, Electrochem. Solid-State Lett., 2008, 11, A64–A67 CrossRef CAS PubMed.
- A. V. Chadwick, S. L. P. Savin, S. Fiddy, R. Alcántara, D. F. Lisbona, P. Lavela, G. F. Ortiz and J. L. Tirado, J. Phys. Chem. C, 2007, 111, 4636–4642 CAS.
- T. Y. Wei, C. H. Chen and C. C. Hu, Adv. Mater., 2010, 22, 347–351 CrossRef CAS PubMed.
- U. M. Patil, J. S. Sohn, S. B. Kulkarni, S. C. Lee, H. G. Park, K. V. Gurav, J. H. Kim and S. C. Jun, ACS Appl. Mater. Interfaces, 2014, 6, 2450–2458 CAS.
- J. Wang, Y. C. Song, Z. S. Li, Q. Liu, J. D. Zhou, X. Y. Jing, M. L. Zhang and Z. H. Jiang, Energy Fuels, 2010, 24, 6463–6467 CrossRef CAS.
- Q. F. Wang, X. F. Wang, B. Liu, G. Yu, X. J. Hou, D. Chen and G. Z. Shen, J. Mater. Chem. A, 2013, 1, 2468–2473 CAS.
- C. Z. Yuan, J. Y. Li, L. R. Hou, X. G. Zhang, L. F. Shen and X. W. Lou, Adv. Funct. Mater., 2012, 22, 4592–4597 CrossRef CAS PubMed.
- G. Q. Zhang, H. B. Wu, H. E. Hoster, M. B. Chan-Park and X. W. Lou, Energy Environ. Sci., 2012, 5, 9453–9456 CAS.
- B. Wang, G. R. Williams, Z. Chang, M. Jiang, J. F. Liu, X. D. Lei and X. M. Sun, ACS Appl. Mater. Interfaces, 2014, 6, 16304–16311 CAS.
- C. H. Wang, X. Zhang, D. C. Zhang, C. Yao and Y. W. Ma, Electrochim. Acta, 2012, 63, 220–227 CrossRef CAS PubMed.
- V. Gupta, S. Gupta and N. Miura, J. Power Sources, 2008, 175, 680–685 CrossRef CAS PubMed.
- W. H. Chen, Y. F. Yang, H. X. Shao and J. Fan, J. Phys. Chem. C, 2008, 112, 17471–17477 CAS.
- Y. Li, X. W. Xie, J. L. Liu, M. Cai, J. Rogers and W. J. Shen, Chem. Eng. J., 2008, 136, 398–408 CrossRef CAS PubMed.
- J. J. Tunney and C. Detellier, Clays Clay Miner., 1994, 42, 552–560 CAS.
- A. Kasai and S. Fujihara, Inorg. Chem., 2006, 45, 415–418 CrossRef CAS PubMed.
- T. Stanimirova and T. Hibino, Appl. Clay Sci., 2006, 31, 65–75 CrossRef CAS PubMed.
- J. C. Villegas, O. H. Giraldo, K. Laubernds and S. L. Suib, Inorg. Chem., 2003, 42, 5621–5631 CrossRef CAS PubMed.
- X. H. Liu, R. Z. Ma, Y. Bando and T. Sasaki, Adv. Mater., 2012, 24, 2148–2153 CrossRef CAS PubMed.
- J. J. Deng, J. C. Deng, Z. L. Liu, H. R. Deng and B. Liu, J. Mater. Sci., 2009, 44, 2828–2835 CrossRef CAS.
- Q. Wang and D. O’ Hare, Chem. Rev., 2012, 112, 4124–4155 CrossRef CAS PubMed.
- N. Thomas and M. Rajamathi, Langmuir, 2009, 25, 2212–2216 CrossRef CAS PubMed.
- C. Wang, Y. Zhou, M. Y. Ge, X. B. Xu, Z. L. Zhang and J. Z. Jiang, J. Am. Chem. Soc., 2010, 132, 46–47 CrossRef CAS PubMed.
- Y. Wang, Z. Q. Shi, Y. Huang, Y. F. Ma, C. Y. Wang, M. M. Chen and Y. S. Chen, J. Phys. Chem. C, 2009, 113, 13103–13107 CAS.
| This journal is © The Royal Society of Chemistry 2015 |