DOI:
10.1039/C5RA10166B
(Paper)
RSC Adv., 2015,
5, 62469-62476
A novel approach for spectrophotometric determination of succinylcholine in pharmaceutical formulation via host–guest complexation with water-soluble p-sulfonatocalixarene†
Received
1st June 2015
, Accepted 13th July 2015
First published on 13th July 2015
Abstract
Succinylcholine (SUC) is a quaternary ammonium neuromuscular blocking agent. Direct determination of SUC in bulk drugs and formulations is a challenging analytical task due to the lack of a detectable chromophore and sensitive detection techniques. We have exploited both the strong UV absorbance of p-sulfonatocalix[4]arene (SCX4) and its outstanding complexation properties towards quaternary ammonium compounds to determine SUC. The characteristics of a host–guest complexation between SCX4 and SUC were investigated using UV and 1H NMR spectroscopy. The Job's plot analysis reveals a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the host–guest complex with a binding affinity Ka of 7.8 × 104 L mol−1. This novel method is based on spectrophotometric measurement of the formed complex peak after resolving the overlap from the host SCX4 spectrum and was used for the quantitation of SUC. The linear range was found to be from 1.0 × 10−5 to 18.0 × 10−5 mol L−1 with a detection limit of 7.3 × 10−6 mol L−1 (2.88 μg mL−1). This method is straightforward and shows high sensitivity. Moreover, it was successfully employed to determine SUC in pharmaceutical formulation. Subsequent statistical analysis of the obtained results and comparison with the official US pharmacopeial benchmark yielded favorable results.
1 stoichiometry of the host–guest complex with a binding affinity Ka of 7.8 × 104 L mol−1. This novel method is based on spectrophotometric measurement of the formed complex peak after resolving the overlap from the host SCX4 spectrum and was used for the quantitation of SUC. The linear range was found to be from 1.0 × 10−5 to 18.0 × 10−5 mol L−1 with a detection limit of 7.3 × 10−6 mol L−1 (2.88 μg mL−1). This method is straightforward and shows high sensitivity. Moreover, it was successfully employed to determine SUC in pharmaceutical formulation. Subsequent statistical analysis of the obtained results and comparison with the official US pharmacopeial benchmark yielded favorable results.
1. Introduction
Succinylcholine is a quaternary ammonium neuromuscular depolarizing blocking agent. It blocks the effects of the neurotransmitter acetylcholine in skeletal muscle, resulting in the paralysis of voluntary muscles and the diaphragm. Rapid onset (less than 1 minute) makes SUC a good choice in emergency situations. It is used as an adjunct to anesthesia to induce skeletal muscle relaxation. Succinylcholine is an analytically challenging compound because it is difficult to monitor since it does not absorb in the UV band (has no detectable chromophore), does not present fluorescence, is not redox electroactive, and is difficult to derivatize.
Reviewing the literature reveals that most of the analytical methods previously reported for the determination of SUC have used HPLC coupled with mass spectrometry (MS)1,2 or electrochemical detection.3,4 Furthermore, capillary electrophoresis (CE) with indirect UV detection,5–7 contactless conductivity detection8 and CE coupled with attenuated total internal reflectance infrared microspectroscopy (FT-IR)9 has been reported. However, due to the lack of a detectable chromophore, no spectrophotometric method for determining SUC in a pharmaceutical formulation has been described in the literature. Our motivations for developing a new spectrophotometric method of analysis are thus twofold: the achievement of better performance parameters and the development of a fast, simple and highly sensitive method to efficiently detect SUC.
The advances of host–guest chemistry has matured sufficiently to have utility in many interesting applications and remains a fruitful area for research. Calixarenes represent a particularly significant class of host molecules and have been widely exploited in all areas in supramolecular chemistry. They are described as ‘macrocycles with unlimited possibilities’ for their facile modification.
Among the various calixarene derivatives, the chemistry of p-sulfonatocalixarenes is fascinating for their high water solubility (>0.1 mol L−1),10 three-dimensional, flexible, π-rich cavities, and their ability to provide additional anchoring points of sulfonate groups, which endows them with versatile inclusion/complexation properties for different kinds of guest molecules. Owing to these pronounced inclusion properties, the ionic/molecular recognition based on p-sulfonatocalixarenes has been widely investigated in many fields, including crystal engineering, biochemistry, sensor/probe, and catalysis.
The aim of the present work is to develop and validate the first spectrophotometric method for the determination of SUC. We have explored the host–guest complexation between SCX4 with SUC in aqueous medium. Moreover, the characterization of the formed complex has been performed by means of UV and 1H NMR spectroscopy. The stoichiometry and binding constant were determined directly from Job's plot. In order to measure the UV spectrum of the complex in the presence of the overlapped SCX4 host spectrum, derivative ratio method was adopted to resolve this overlap. The theoretical linear relation between the concentration of the analyte SUC and the formed complex was confirmed by the experimental results. Furthermore, the proposed method was applied for the quantitation of SUC in commercially available pharmaceutical products with favorable results compared to the official U.S pharmacopeial benchmark.
2. Experimental
2.1. Chemicals and reagents
Succinylcholine chloride (SUC) and 4-sulfocalix[4]arene (SCX4) were purchased from Sigma-Aldrich (Steinheim, Germany). Deionized bi-distilled water supplied by Egypt Otsuka pharmaceutical company (Cairo, Egypt) was used for all sample solution preparations. All solutions were refrigerated at 4 °C until used. SUCCINYLCHOLINE CHLORIDE® injection (U.S.P. 27) manufactured by Misr Company for pharmaceutical and chemicals industries (Cairo, Egypt). Batch no. 525032 labeled to contain 20 mg of SUC per 1 mL.
2.2. Instruments
UV spectrophotometric measurements were carried out with SHIMADZU dual beam UV-visible spectrophotometer (Kyoto, Japan), model UV-1650. 1H NMR spectra were measured using an Bruker Ascend™-400/R−1 MHz spectrometer.
2.3. Procedures
2.3.1 Construction of calibration graph (DD1 method). Into a series of 10 mL volumetric flasks, various amounts of SUC stock standard solution (1 × 10−4 mol L−1) were added to a constant volume (2.0 mL) of SCX4 stock solution (1 × 10−3 mol L−1) and the volume was completed with distilled water. The zero order spectra of the prepared solutions were measured then divided by the spectrum of 2 × 10−4 mol L−1 SCX4, and the first derivative of the ratio spectra (DD1) were obtained using a scaling factor of 10 and Δλ = 4 nm. The peak amplitudes of the first derivative of the ratio spectra were measured at 315 nm. A calibration graph relating the peak amplitudes of (1DD315) to the corresponding concentrations of SUC was constructed, and the corresponding regression equation was computed.
2.3.2 Determination of SUC in pharmaceutical formulation. 1.0 mL SUCCINYLCHOLINE CHLORIDE® injection (U.S.P. 27) was transferred into a 50 mL volumetric flask and filled to the mark with bi-distilled water. The concentration of this prepared sample was 1.0 × 10−3 mol L−1. 0.5 mL of this solution was accurately transferred to a 10 mL volumetric flask containing (2.0 mL) of SCX4 stock solution (1 × 10−3 mol L−1) and completed to mark with distilled water. Then the procedure was completed as described under construction of calibration graph.
3. Results and discussion
The possible applications of calixarenes in analytical chemistry are seemingly endless. Water-soluble calix[n]arenesulfonates have been studied for their ability to bind to several dye molecules,11,12 including native amino acids,13,14 pesticides,15 several specific drugs and their intermediates.16–18
3.1. Spectroscopic characterization of the complex between SUC and SCX4
3.1.1 UV-spectroscopic studies. To evaluate the formation of an inclusion complex by UV-spectroscopy, Fig. 1 shows the UV spectra of SUC, SCX4 and mixture of SUC and SCX4 each 2 × 10−4 mol L−1 in distilled water. The spectrum of SUC (blue curve) shows weak absorbance and a peak at 206 nm, while the free SCX4 (black curve) shows strong UV absorbance with a characteristic pair of absorption maxima near 276 and 283 nm. The spectrum of the mixture (red curve) shows a merge of the two absorption maxima characteristic of free SCX4 and appearance of peak at 282 nm. The observed spectrum change is probably due to the formation of a host–guest inclusion complex between SCX4 and SUC. To further examine the complex structure, 1H NMR spectroscopic studies were performed.
 |
| | Fig. 1 Absorbance spectra of 2 × 10−4 mol L−1 SUC (blue curve), 2 × 10−4 mol L−1 SCX4 (black curve) and a mixture containing equimolar concentration (2 × 10−4 mol L−1) of both SUC and SCX4 (red curve) in distilled water. | |
3.1.2 1H NMR spectroscopic studies. The 1H NMR spectra of the complex of SUC with SCX4 was measured in D2O to provide unambiguous evidence for the accommodation of this quaternary ammonium analyte into the cavity of SCX4. Fig. 2 shows the 1H NMR spectra of the guest SUC alone, (Fig. 2a), and with 1 equiv. amount of SCX4 (Fig. 2b). The SUC guest protons experienced large complexation-induced upfield shift (CIS), owing to the shielding effect from the aromatic wall of SCX4, data are summarized in Table 1. The signal for the protons of the methyl groups attached to the quaternary ammonium head of SUC, Ha, displayed the largest upfield shift (∼0.8 ppm) indicating that they experienced the greatest shielding effect. The implication of this data was that SUC was included into the calixarene cavity via its N-terminal moiety. This was supported by the fact that Δδa was greater than Δδb,c,d which indicated that protons Ha were inserted further into the cavity than Hb, Hc and Hd. While the signal for the protons of the two methylene groups, Hd, displayed the smallest upfield shift (∼0.1 ppm) indicating the need of the oxygen atoms of SUC to stick out of the apolar SCX4 cavity in order to be exposed to polar medium. Therefore the mode of inclusion presented in Scheme 1 was proposed for the SCX4–SUC complex. With respect to the SCX4 protons, the signals of the aryl and methylene bridges protons do not shift appreciably upon inclusion of SUC (Δδ ∼ 0.001 ppm), but the signal of the methylene bridges protons broaden to the baseline (see Fig. S-1, ESI† for the NMR spectrum of SCX4 alone). This is characteristic for a complexation-induced conformational rigidification of calixarenes.19 Subsequently, integration of the guest and host signals at this stage suggested the complex has a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (host/guest) ratio. In order to further study the stoichiometry and the stability constant of this supramolecular complex, Job's plot was performed.
1 (host/guest) ratio. In order to further study the stoichiometry and the stability constant of this supramolecular complex, Job's plot was performed.
 |
| | Fig. 2 1H NMR spectra of SUC alone (2a), and SUC with 1 equiv. amount of SCX 4 (2b). The structure of SUC is also shown with appropriate protons labeled letters. | |
Table 1 Shieldings (ppm) observed for SUC guest protons upon complexation with SCX4 host
| Respective protons signala,b |
Free guest |
Complexed guest |
Assignments of signals to the respective protons. The integration ratio between the proton signals of SUC and the respective protons of SCX4 indicates that the stoichiometry of the complex is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (guest 1 (guest![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) host). host). |
| (Ha) |
(CH3)3N+ |
3.2 |
2.4 |
| (Hb) |
CH2N+ |
3.7 |
3.2 |
| (Hc) |
CH2O |
4.6 |
4.3 |
| (Hd) |
CH3 |
2.8 |
2.7 |
 |
| | Scheme 1 Suggested structure of inclusion-complex of SUC guest and SCX4 host. | |
3.2. Job's plot by UV-spectroscopy
Generally, determination of the stoichiometry of chemical equilibrium reactions have been performed using several methods such as the method of continuous variation (Job's plot),20,21 slope ratio method,22 and mole ratio method.23 In this paper we have chosen Job's method due to its simplicity. A series of solutions of different molar ratio of SCX4 and SUC between 0 and 1 were prepared such that the sum of the total concentration C = Ch + Cg remained constant at (1 mM), then the UV-spectra of the solutions series were measured. Due to the severe overlap between the host SCX4 peak and the complex peak which hinders the direct determination of the absorbance of complex. Derivative ratio method has been adopted to resolve the overlap. Derivative ratio spectrophotometry is an analytical technique of great utility for resolving bands of overlapped binary24 and ternary mixtures25 without previous separation. The main advantage of the method is that the whole spectrum of interfering host SCX4 is cancelled. And hence the choice of the wavelength used for calibration is not critical compared to ordinary derivative methods.26 Practically, the derivative ratio method was performed by dividing spectra of the prepared solutions by the spectrum of 2 × 10−4 M SCX4, Fig. S-2 and S-3 (ESI†), and obtaining the first derivative of the ratio spectra (1DD) using scaling factor 10 and Δλ = 4 nm, then measuring the amplitudes of the first derivative peaks of ratio spectra at 315 nm (1DD315) as shown in Fig. S-3 (ESI†). These peak amplitudes are proportional to the complex concentration.
The modified Job's plot was developed where the peak amplitudes (S) at 315 nm is plotted as y-coordinate versus the corresponding host SCX4 molar fraction (x) as a x-coordinate. A curve with a maximum for molar fraction of 0.5 is obtained as shown in Fig. 3. Consequently, two straight lines were traced directly on the experimental points and intersected at a point from which the stoichiometry of the complex is determined and confirmed to be (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
 |
| | Fig. 3 Job's plot for the determination of the stoichiometry of SCX4 and SUC in the complex, [SUC] + [SCX4] = 1.0 × 10−3 mol L−1. | |
In a similar way, the stability constant of the complex was estimated from the vertical distance that separates the intersection point of the straight lines plotted and the maximum of the experimental curve using the following equation.27
where
K is the stability constant,
C represents the total concentration of the complex,
m and
n are the corresponding stoichiometric coefficients of the guest and host respectively,
α is the degree of dissociation of the complex which is obtained by the following equation:
where
Smax is the peak amplitude of the maximum at the experimental curve and
SIP is the peak amplitude corresponding to the intersection point of the straight lines. The experiment was discussed in details in the (ESI) Fig. S-4.
† From the results, the binding constant was 7.8 × 10
4 L mol
−1, thus confirming a high affinity between SCX4 and SUC.
For further confirmation of the stoichiometry of the formed complex and the value of the binding constant, we applied a recently published method for the interpretation of Job's plot by normalizing the measured peak amplitudes (S) at each point to the maximum value of peak amplitude at mole fraction 0.5 (Smax), and determining the sum of the normalized values (∑S/Smax). It was experimentally found to be 6.01 which are consistent with the theoretically predicted values for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 association complex and the value of binding constant.21 The experiment was discussed in details in the (ESI) Fig. S-5 and Table S-1.†
1 association complex and the value of binding constant.21 The experiment was discussed in details in the (ESI) Fig. S-5 and Table S-1.†
3.3. Theoretical relation between the concentrations of SUC and the inclusion complex formed
Based on the results that the host (H) SCX4 and the guest SUC (G) forms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio complex (HG), the following expression can be written as
1 ratio complex (HG), the following expression can be written as
The formation constant of the complex (K) is given by
| |
 | (2) |
where [H] = [H]
o − [HG] and [G] = [G]
o − [HG], and [H]
o and [G]
o denote the initial concentrations of host SCX4 and guest SUC, respectively.
| |
 | (3) |
If [H]o ≫ [G]o, then [H]o − [HG] ≈ [H]o
| |
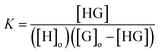 | (4) |
By rearrangement;
| |
 | (5) |
| |
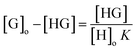 | (6) |
| |
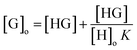 | (7) |
| |
 | (8) |
Based on the assumptions that the concentration [H]o is kept constant during all experimental measurements and [H]o is much higher than [G]o, then based on eqn (8) the [G]o is linearly proportional to [HG] because (1 + 1/HoK) is constant.
In practice and in order to verify the theoretical assumption, fixed high concentration of SCX4 (2.0 × 10−4 mol L−1) was added to various lower concentrations of SUC, Fig. 4, and applying the derivative ratio method as above (ESI Fig. S-6†), then the peak amplitudes at 315 nm (1DD315) of the complex formed was plotted against SUC concentration as shown in Fig. 5. The relationship between the complex peak amplitudes and SUC concentration are shown in Fig. 6. It is apparent the there is a linearity relation in the range of 1 × 10−5–18 × 10−5 mol L−1 SUC, until SUC concentrations approach the maximum limiting value (20 × 10−5 mol L−1), indicating the combining ratio. Once the amount of SUC exceeds the stoichiometrically required amount, a plateau was observed indicating that SCX4 becomes the limiting reactant and the amount of the formed complex remains constant as shown in Fig. S-7 (ESI†). This relation is similar to the behavior observed in the molar ratio method.28
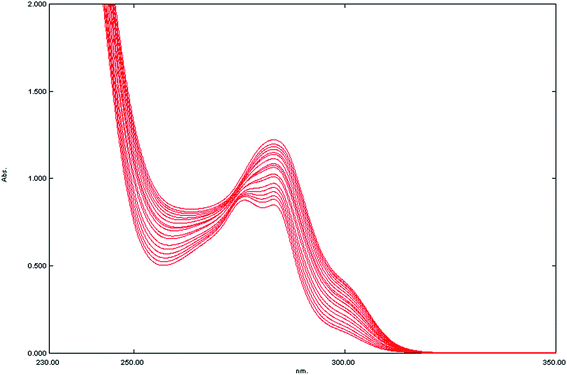 |
| | Fig. 4 Absorbance spectra for mixtures containing various concentrations of SUC in the range of (1 × 10−5–18 × 10−5 mol L−1) and a fixed concentration (2 × 10−4 mol L−1) of SCX4 in distilled water. | |
 |
| | Fig. 5 First derivative of ratio spectra of SUC (1 × 10−5–18 × 10−5 mol L−1) using the spectrum of 2 × 10−4 mol L−1 of SCX4 as a divisor. | |
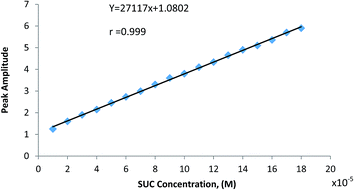 |
| | Fig. 6 Plot of peak amplitude of (SCX4–SUC) host–guest complex at 315 nm vs. the concentration of SUC. | |
3.4. Application to pharmaceutical formulation
In order to demonstrate the applicability of the spectrophotometric method to real samples, quantitation of SUC was achieved on commercially available pharmaceutical product; SUCCINYLCHOLINE CHLORIDE® injection (U.S.P. 27). Results obtained by the proposed procedures for the determination of analyte in commercial dosage form were statistically compared to those of the official U.S. Pharmacopeia (USP) method29 (HPLC method using 10% of 1 N aqueous tetramethylammonium chloride in methanol, pH: 3.0 adjusted with HCl, UV detection at 214 nm). The data are summarized in Table 2. From these results we can conclude that no significant difference was observed between the proposed method and the pharmacopeial method. Moreover, the proposed spectrophotometric method is simple, less time-consuming, thus lowering analysis time and cost per sample.
Table 2 Determination of SUC in pharmaceutical formulation by the proposed spectrophotometric and the official method29
| Pharmaceutical formulation |
Recovery% ± S.D.a of SUC |
| This work |
Official methodb |
| Average of five determinations. HPLC method using 10% of 1 N aqueous tetramethylammonium chloride in methanol, pH: 3.0 adjusted with HCl, UV detection at 214 nm. The values in parentheses are the corresponding theoretical values for t and F at P = 0.05. |
| SUCCINYLCHOLINE CHLORIDE® injection (U.S.P. 27) (20 mg mL−1) |
100.19 ± 1.232 |
99.42 ± 1.114 |
| t-Testc |
1.124 (2.306) |
| Fc |
1.11 (6.39) |
3.5. Method validation
Method validation was performed according to ICH guidelines30 with respect to linearity, accuracy, precision, and robustness for the proposed method. Table 3 shows results of accuracy, repeatability, and intermediate precision of the method. The linear regression equation is: Y = 27117x + 1.0802, with a correlation coefficient of 0.999 (S.D. = 1.635, n = 18). The limit of detection (LOD) is 7.3 × 10−6 mol L−1 (2.89 μg mL−1), which is given by the equation LOD = 3.3 σ/S. Here σ is the standard deviation of the blank measurement (n = 5) and S is the slope of the calibration curve. High values of correlation coefficient and small value of intercept validated the linearity of the calibration graph and in accordance with Beer's law. The RSD values indicated the high reproducibility of the proposed method. From the results obtained, we concluded that the suggested spectrophotometric method could be considered sensitive, accurate and reproducible for the determination of SUC over the tested range.
Table 3 Assay parameters and method validation sheeta
| Parameter |
The proposed spectrophotometric method |
| RSDa%, RSDb% the intra-day, inter-day respectively (n = 5) relative standard deviation of concentrations 4 × 10−5, 8 × 10−5 and 12 × 10−5 mol L−1 of SUC by the proposed spectrophotometric method. |
| Range (mol L−1) |
1.0 × 10−5–18 × 10−5 |
| Slope |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117 117 |
| Intercept |
1.0802 |
| Mean |
100.90 |
| S.D. |
1.635 |
| Variance |
2.673 |
| Corr. coeff. (r) |
0.999 |
| Coeff. of variation |
1.620 |
| RSDa% |
1.085 |
| RSDb% |
1.054 |
| LOD |
7.3 × 10−6 mol L−1 |
| LOQ |
2.9 × 10−5 mol L−1 |
4. Conclusions
This paper has presented a novel method for determining SUC analyte, which has no detectable chromophore, using a simple spectrophotometric method. The work exploited a host–guest complexation in aqueous medium between SCX4, which has both strong UV-absorbance and outstanding complexation properties towards quaternary ammonium compounds such as SUC. The host–guest complex was studied and characterized by means of UV and 1H NMR spectroscopy. The stability constant and the binding ratio of complexation were estimated to be 7.8 × 104 L mol−1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, using Job's plot. The peak amplitude of the host–guest complex formed was found to be linearly proportional to the concentration of the analyte SUC in the range of 1.0 × 10−5–18 × 10−5 mol L−1. The method has been validated according to ICH guidelines and the limit of detection was calculated to be 7.3 × 10−6 mol L−1 (2.89 μg mL−1). Moreover, this method has been applied for the determination of SUC in pharmaceutical formulation without statistical difference from the official U.S. Pharmacopeial (USP) method. In conclusion, the proposed spectrophotometric method is simple, efficient, less time-consuming and shows high sensitivity compared to other published HPLC and CE methods. As such, it can be used for quality control and routine analysis of SUC. In general, the spectrophotometric method presented here is not limited to the determination of SUC as it opens a new avenue for the determination of small compounds that possess quaternary ammonium groups and that lack a detectable chromophore.
1, respectively, using Job's plot. The peak amplitude of the host–guest complex formed was found to be linearly proportional to the concentration of the analyte SUC in the range of 1.0 × 10−5–18 × 10−5 mol L−1. The method has been validated according to ICH guidelines and the limit of detection was calculated to be 7.3 × 10−6 mol L−1 (2.89 μg mL−1). Moreover, this method has been applied for the determination of SUC in pharmaceutical formulation without statistical difference from the official U.S. Pharmacopeial (USP) method. In conclusion, the proposed spectrophotometric method is simple, efficient, less time-consuming and shows high sensitivity compared to other published HPLC and CE methods. As such, it can be used for quality control and routine analysis of SUC. In general, the spectrophotometric method presented here is not limited to the determination of SUC as it opens a new avenue for the determination of small compounds that possess quaternary ammonium groups and that lack a detectable chromophore.
Acknowledgements
This manuscript is dedicated to the memory of deceased Professor M. Galal El-Bardicy, whose long interest in spectrophotometric methods inspired the rest of us in its pursuit. Furthermore, we would like to thank Professor Philippe Buhlmann (Chemistry department, University of Minnesota, USA) for providing ref. 21 with all the fruitful discussions.
References
- H. Tsutsumi, M. Nishikawa, M. Katagi and H. Tsuchihashi, J. Health Sci., 2003, 49, 285–291 CrossRef CAS.
- J. J. Roy, D. Boismenu, H. Gao, O. A. Mamer and F. Varin, Anal. Biochem., 2001, 290, 238–244 CrossRef CAS PubMed.
- S. Chen, V. Soneji and J. Webster, J. Chromatogr. A, 1996, 739, 351–357 CrossRef CAS PubMed.
- H. Gao, S. Roy, F. Donati and F. Varin, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 718, 129–134 CrossRef CAS.
- R. Schoftner, W. Buchberger and H. Malissa, J. Chromatogr. A, 2001, 920, 333–344 CrossRef CAS PubMed.
- M. J. van der Schans, J. C. Reijenga and F. M. Everaerts, J. Chromatogr. A, 1996, 735, 387–393 CrossRef CAS PubMed.
- R. Koike, F. Kitagawa and K. Otsuka, J. Chromatogr. A, 2007, 1139, 136–142 CrossRef CAS PubMed.
- S. Nussbaumer, S. Fleury-Souverain, S. Rudaz, P. Bonnabry and J. L. Veuthey, J. Pharm. Biomed. Anal., 2009, 49, 333–337 CrossRef CAS PubMed.
- B. M. Patterson, N. D. Danielson and A. J. Sommer, Anal. Chem., 2004, 76, 3826–3832 CrossRef CAS PubMed.
- M. Strobel, K. Kita-Tokarczyk, A. Taubert, C. Vebert, P. A. Heiney, M. Chami and W. Meier, Adv. Funct. Mater., 2006, 16, 252–259 CrossRef CAS.
- Y. Liu, B. H. Han and Y. T. Chen, J. Org. Chem., 2000, 65, 6227–6230 CrossRef CAS PubMed.
- S. Shinkai, S. Mori, H. Koreishi, T. Tsubaki and O. Manabe, J. Am. Chem. Soc., 1986, 108, 2409–2416 CrossRef CAS PubMed.
- G. Arena, A. Contino, F. G. Gulino, A. Magrì, F. Sansone, D. Sciotto and R. Ungaro, Tetrahedron Lett., 1999, 40, 1597–1600 CrossRef CAS.
- F. Sansone, S. Barboso, A. Casnati, D. Sciotto and R. Ungaro, Tetrahedron Lett., 1999, 40, 4741–4744 CrossRef CAS.
- X.-P. Ding, D.-B. Tang, T. Li, S.-F. Wang and Y.-Y. Zhou, Spectrochim. Acta, Part A, 2011, 81, 44–47 CrossRef CAS PubMed.
- J. S. Millership, J. Inclusion Phenom. Macrocyclic Chem., 2001, 39, 327–331 CrossRef CAS.
- Y. Zhou, Q. Lu, C. Liu, S. She and L. Wang, Spectrochim. Acta, Part A, 2006, 63, 423–426 CrossRef PubMed.
- Y. Zhou, Q. Lu, C. Liu, S. She and L. Wang, Anal. Chim. Acta, 2005, 552, 152–159 CrossRef CAS.
- D.-S. Guo, V. D. Uzunova, X. Su, Y. Liu and W. M. Nau, Chem. Sci., 2011, 2, 1722–1734 RSC.
- C. Schalley, Analytical Methods in Supramolecular Chemistry, Wiley-VCH Verlag GmbH, Germany, 2007 Search PubMed.
- E. J. Olson and P. Buhlmann, J. Org. Chem., 2011, 76, 8406–8412 CrossRef CAS PubMed.
- A. E. Harvey and D. L. Manning, J. Am. Chem. Soc., 1950, 72, 4488–4493 CrossRef CAS.
- J. H. Yoe and A. L. Jones, Ind. Eng. Chem., Anal. Ed., 1944, 16, 111–115 CrossRef CAS.
- M. Salem, A. M. El-Kosasy, M. G. El-Bardicy and M. K. Abd El-Rahman, Drug Test. Anal., 2010, 2, 225–233 CAS.
- J. Karpińska, Talanta, 2004, 64, 801–822 CrossRef PubMed.
- M. Nebsen, M. K. Abd El-Rahman, M. Y. Salem, A. M. El-Kosasy and M. G. El-Bardicy, Drug Test. Anal., 2011, 3, 221–227 CrossRef CAS PubMed.
- J. M. Bosque-Sendra, E. Almansa-Lopez, A. M. Garci-Campana and L. Cuadros-Rodriguez, Anal. Sci., 2003, 19, 1431–1439 CrossRef CAS PubMed.
- K. Momoki, J. Sekino, H. Sato and N. Yamaguchi, Anal. Chem., 1969, 41, 1286–1299 CrossRef CAS.
- http://www.pharmacopeia.cn/v29240/usp29nf24s0_m78520.html, U. S. Pharmacopeia, 2011, p. 1404.
- ICH, Q2A (R1) Validation of Analytical Procedures: Text and Methodology, International Conference on Harmonisation, http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf, http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf, November 2005.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra10166b |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the host–guest complex with a binding affinity Ka of 7.8 × 104 L mol−1. This novel method is based on spectrophotometric measurement of the formed complex peak after resolving the overlap from the host SCX4 spectrum and was used for the quantitation of SUC. The linear range was found to be from 1.0 × 10−5 to 18.0 × 10−5 mol L−1 with a detection limit of 7.3 × 10−6 mol L−1 (2.88 μg mL−1). This method is straightforward and shows high sensitivity. Moreover, it was successfully employed to determine SUC in pharmaceutical formulation. Subsequent statistical analysis of the obtained results and comparison with the official US pharmacopeial benchmark yielded favorable results.
1 stoichiometry of the host–guest complex with a binding affinity Ka of 7.8 × 104 L mol−1. This novel method is based on spectrophotometric measurement of the formed complex peak after resolving the overlap from the host SCX4 spectrum and was used for the quantitation of SUC. The linear range was found to be from 1.0 × 10−5 to 18.0 × 10−5 mol L−1 with a detection limit of 7.3 × 10−6 mol L−1 (2.88 μg mL−1). This method is straightforward and shows high sensitivity. Moreover, it was successfully employed to determine SUC in pharmaceutical formulation. Subsequent statistical analysis of the obtained results and comparison with the official US pharmacopeial benchmark yielded favorable results.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (host/guest) ratio. In order to further study the stoichiometry and the stability constant of this supramolecular complex, Job's plot was performed.
1 (host/guest) ratio. In order to further study the stoichiometry and the stability constant of this supramolecular complex, Job's plot was performed.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (guest
1 (guest![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) host).
host).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 association complex and the value of binding constant.21 The experiment was discussed in details in the (ESI) Fig. S-5 and Table S-1.†
1 association complex and the value of binding constant.21 The experiment was discussed in details in the (ESI) Fig. S-5 and Table S-1.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio complex (HG), the following expression can be written as
1 ratio complex (HG), the following expression can be written as

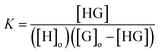

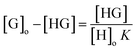
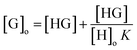




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117
117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, using Job's plot. The peak amplitude of the host–guest complex formed was found to be linearly proportional to the concentration of the analyte SUC in the range of 1.0 × 10−5–18 × 10−5 mol L−1. The method has been validated according to ICH guidelines and the limit of detection was calculated to be 7.3 × 10−6 mol L−1 (2.89 μg mL−1). Moreover, this method has been applied for the determination of SUC in pharmaceutical formulation without statistical difference from the official U.S. Pharmacopeial (USP) method. In conclusion, the proposed spectrophotometric method is simple, efficient, less time-consuming and shows high sensitivity compared to other published HPLC and CE methods. As such, it can be used for quality control and routine analysis of SUC. In general, the spectrophotometric method presented here is not limited to the determination of SUC as it opens a new avenue for the determination of small compounds that possess quaternary ammonium groups and that lack a detectable chromophore.
1, respectively, using Job's plot. The peak amplitude of the host–guest complex formed was found to be linearly proportional to the concentration of the analyte SUC in the range of 1.0 × 10−5–18 × 10−5 mol L−1. The method has been validated according to ICH guidelines and the limit of detection was calculated to be 7.3 × 10−6 mol L−1 (2.89 μg mL−1). Moreover, this method has been applied for the determination of SUC in pharmaceutical formulation without statistical difference from the official U.S. Pharmacopeial (USP) method. In conclusion, the proposed spectrophotometric method is simple, efficient, less time-consuming and shows high sensitivity compared to other published HPLC and CE methods. As such, it can be used for quality control and routine analysis of SUC. In general, the spectrophotometric method presented here is not limited to the determination of SUC as it opens a new avenue for the determination of small compounds that possess quaternary ammonium groups and that lack a detectable chromophore.




