A facile approach for achieving an effective dual sorption ability of Si/SH/S grafted sodium montmorillonite†
Abstract
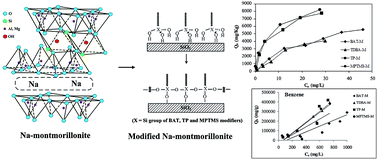
In this study, sodium montmorillonite was functionalized with SH, S and Si functional groups using four different soil modifiers, 1,3-bis(3-aminopropyl)-1,1,3,3-tetramethyldisiloxane [BAT], 3,3′-tetrathiobis(propyl-triethoxysilane) [TP], thiodiglycol bis(3-aminocrotonate) [TDBA] and (3-mercaptopropyl)trimethoxysilane [MPTMS], for the effective uptake of both organic and inorganic pollutants. The uptake of inorganic (Cu2+, Zn2+) and organic [benzene, toluene, ethylbenzene and p-xylene (BTEX)] pollutants was studied with individual as well as binary mixtures. Based on the experimental results, the soil modifier MPTMS best improved the sorption characteristics and, among two metal ions, Cu2+ showed enhanced adsorption. Sorption of BTEX was not correlated with a single parameter and hence it differed for the different cases based on the organic matter content. The obtained log KOC and log KOM values of BTEX in this study for modified montmorillonite are comparatively larger than those of unmodified montmorillonite or natural soil. Sorption carried out in the binary mixtures showed that there is no interaction between these pollutants and the presence of one did not retard the adsorption of another. The uptake phenomenon was influenced by various combined factors such as the nature, surface charge and surface area of the modified soils.

 Please wait while we load your content...
Please wait while we load your content...