Correlating experimental electrochemistry and theoretical calculations in 2′-hydroxy chalcones: the role of the intramolecular hydrogen bond†
Abstract
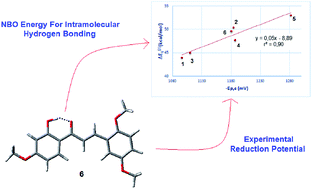
In this work we present a study on the molecular structure and electrochemical behavior of a series of methoxylated 2′-hydroxychalcones, whose antitumor activity has been previously described. Cyclic voltammetry was used to quantitatively characterize the formation and stability of the anion radicals. The molecular structure of the neutral compounds and their anion radicals, particularly the intramolecular hydrogen bonds (IHB), were investigated through density functional theory (DFT) calculations. Geometrical and frontier orbital changes in the anion, relative to the neutral species, were examined and the adiabatic and vertical electron affinities (AEA, VEA) as well as vertical detachment energy (VDE) were calculated. Natural bond orbital (NBO) analysis was used to obtain insights into the electronic characteristics of the IHB and the results were correlated with 1H-NMR chemical shifts. A direct relation among the substitution pattern on rings A and B, the strength of the IHBs and the reduction potentials was found. NBO energies (ΔE(2)ij) show that the main contributions to the stabilization of the IHBs arises from LP → σ* interactions. The strength of IHBs, given by ΔE(2)ij, exhibit a notable quantitative correlation with the experimental reduction potential, which, at least to the best of our knowledge, has not been described before for any type of molecule. The results show the importance of the methoxy substitution pattern on the IHB and redox properties of these compounds. Our findings have potential implications in the design of antitumor chalcones.

 Please wait while we load your content...
Please wait while we load your content...