Photo-catalytic deactivation of sulfate reducing bacteria – a comparative study with different catalysts and the preeminence of Pd-loaded WO3 nanoparticles
Abstract
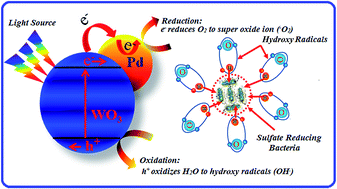
Sulfate reducing bacteria (SRB), predominantly present in the produced water in oil fields is known for being an agent for degrading the quality of crude oil by introducing an elevated level of sulfur content, initiating oil souring and corroding the oil pipelines. In addition, this bacterium poses an immense health threat to the oil field workers due to the generation of radioactive barium sulfide. Photo-catalytic deactivation of the sulfate reducing bacteria in water with four different pure and palladium loaded photo-catalysts was carried out and their relative efficiencies are compared. It was found that n-Pd/WO3 at an optimum concentration, in conjunction with 355 nm pulsed laser radiation showed a substantial increase in the photo-catalytic deactivation of SRB in contaminated water. A 110 fold increase in the SRB deactivation rate, compared to UV radiation (in the absence of catalyst) and a 30 fold increase in the same, compared to pure WO3, and the bench mark catalyst (TiO2) under the same experimental conditions was observed. All the nano-structured photo-catalysts were synthesized, optically and morphologically characterized to optimize the function of each catalyst effective in the deactivation of harmful sulfate-reducing bacteria in water.

 Please wait while we load your content...
Please wait while we load your content...