Synthesis of lipase nano-bio-conjugates as an efficient biocatalyst: characterization and activity–stability studies with potential biocatalytic applications†
Kirtikumar Chandulal Badgujar*a,
Takehiko Sasaki b and
Bhalchandra Mahadeo Bhanage*a
b and
Bhalchandra Mahadeo Bhanage*a
aInstitute of Chemical Technology, Department of Chemistry, Matunga, Mumbai-400019, India. E-mail: bm.bhanage@ictmumbai.edu.in; bm.bhanage@gmail.com; bhalchandra_bhanage@yahoo.com; Fax: +91-22-2414-5614; Tel: +91-22-3361-2601/2222
bDepartment of Complexity Science and Engineering, Graduate School of Frontier Sciences, The University of Tokyo, Chiba 277-8561, Japan
First published on 18th June 2015
Abstract
In the present study, we have synthesized lipase-nano-bio-conjugates via immobilization of various lipases on multiwall carbon nano-tubes (MCNT), in order to construct an efficient and recyclable biocatalytic system. In a screening study lipase Pseudomonas fluorescens (PFL) acted as an efficient biocatalyst (lipase-nano-bio-conjugates) which showed higher retention of lipase activity and protein loading. Consequently the immobilization support![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase (MCNT
lipase (MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL) composition was screened in which MCNT
PFL) composition was screened in which MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL (2
PFL (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was calculated as a robust biocatalyst composition which showed higher activity retention and protein loading. This nano-bio-conjugate was then characterized in detail with physical and biochemical techniques using SEM, TEM, FTIR, Km, Vmax, catalytic efficiency and (%) water content analysis. This developed biocatalyst was further used for practical biocatalytic applications such as O-acylation reactions. Various reaction parameters were optimized in detail like reactant molar ratio (2
1) was calculated as a robust biocatalyst composition which showed higher activity retention and protein loading. This nano-bio-conjugate was then characterized in detail with physical and biochemical techniques using SEM, TEM, FTIR, Km, Vmax, catalytic efficiency and (%) water content analysis. This developed biocatalyst was further used for practical biocatalytic applications such as O-acylation reactions. Various reaction parameters were optimized in detail like reactant molar ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5), solvent, MCNT
3.5), solvent, MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL biocatalyst amount (36 mg), temperature (50 °C) etc. The developed biocatalytic protocol was then extended to synthesize several (twenty-two) industrially important acylated moieties with an excellent yield, these products are well characterized by 1HNMR, 13CNMR and GCMS analysis. Moreover in the present study, we have reviewed the potential industrial applications of various synthesized compounds. Also, we have studied the thermodynamic aspect which demonstrated more feasibility of use of immobilized MCNT
PFL biocatalyst amount (36 mg), temperature (50 °C) etc. The developed biocatalytic protocol was then extended to synthesize several (twenty-two) industrially important acylated moieties with an excellent yield, these products are well characterized by 1HNMR, 13CNMR and GCMS analysis. Moreover in the present study, we have reviewed the potential industrial applications of various synthesized compounds. Also, we have studied the thermodynamic aspect which demonstrated more feasibility of use of immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase over free lipase. Interestingly, immobilized MCNT
PFL lipase over free lipase. Interestingly, immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase showed 2.3 fold higher catalytic activity than free PFL. Besides this, the biocatalyst was efficiently recycled for up to five cycles. Thus the present protocol demonstrated, (i) synthesis of nano-bio-conjugates as a bio-catalyst, (ii) detailed physical-biochemical characterization of nano-bio-conjugates, (iii) optimization of the biocatalytic protocol (iv) practical biocatalytic applications along with a mechanistic study (v) a thermodynamic feasibility study and (vi) recyclability study.
PFL lipase showed 2.3 fold higher catalytic activity than free PFL. Besides this, the biocatalyst was efficiently recycled for up to five cycles. Thus the present protocol demonstrated, (i) synthesis of nano-bio-conjugates as a bio-catalyst, (ii) detailed physical-biochemical characterization of nano-bio-conjugates, (iii) optimization of the biocatalytic protocol (iv) practical biocatalytic applications along with a mechanistic study (v) a thermodynamic feasibility study and (vi) recyclability study.
1. Introduction
Enzymes are widely studied bio-molecules in various fields such as bio-chemical engineering, bio-medical sciences, bio-informatics, microbiology, biotechnology, bio-chemistry and Chemistry.1,2 In particular, enzyme lipase {triacyl glycerol hydrolases, (E.C. 3.1.1.3)} from the hydrolases class has been attracted significant consideration in food, flavour, dairy, leather and pharma sectors as a versatile biocatalyst to carry out organic synthesis in different reaction media at mild reaction conditions.2–4 However, potential biocatalytic applications of these free lipase biomolecules are greatly hampered by their sensitive proteomic nature which reflect poor solubility, activity and stability in various organic media.3–5 Moreover, enzymes are active in aqueous media whereas, organic compounds do not show any solubility.3–7The advance skilful immobilization technique is the only way to triumph over above proposed difficulties.2–8 Hence, various researchers are engaged to improve activity, stability and reuse of enzymes in non-aqueous media for effective biocatalytic applications.5–10 Immobilization of enzyme is the most fundamental aspect to improve the sufficient activity and stability of enzymes, moreover immobilization build up a heterogeneous reaction system which can shield the sensitive enzymes from the surrounding reaction media and also overcome the economic reusability issue.5–12 Furthermore, the use of immobilized biocatalytic system has various green-chemistry advantages such as higher activity, recyclability, higher chemo-, regio-, enantio-selectivity, mild reaction condition, sustainable E factor, easy handling, safe synthetic protocol and no environmental hazards/issues.2–12 Till time immobilization of lipases are reported on various types of carriers such as polymeric matrix, polymer beads, gels, sol–gels, fibres, meso-, micro-porous materials etc. via physical adsorption, entrapment, and cross-linking method.3–12 However, an ideal immobilisation of biocatalyst on carrier should retain or improve its catalytic activity, stability and recyclability.2–8
Nano-materials for immobilization and their practical synthetic applications (bio-nonmaterial and nano-biocatalysis) is a fast growing research area which involves the enzyme immobilization on nano-material and their biocatalytic applications.10–19 Different nano-structured materials like nano-particles, nano-fibres, nano-wires, and nano-composites have been engaged as a novel immobilisation support for enzymes.10,12,14,16,18,19 These nano-materials serves as an excellent immobilization material to synthesize various nano-bio-conjugates as compared to bulk solid materials. This is because of key properties such as higher surface area, low mass transfer resistance, effective enzyme loading, nano-scale dispersion and ease of surface functionalization/modification.10–19
Thus, discovery of nano-scale materials proved countless applications and scope based on their extraordinary properties owing to size of nano-materials.10–19 Among various said nano-materials, a special mention goes to carbon based nano-materials such as carbon nano-tubes (CNTs) which have attracted the significant attention in biomedical science and technology.9–19 Terminal-end and side-walls of CNTs can be modified by various chemical, physical and biological methods according to choice of application.11,12 Moreover, CNTs possess wide applications because of excellent properties such as structural, mechanical, thermal and biocompatibility.13,15 Due to these reasons, both single-walled carbon nano-tube (SCNTs) and multi-walled carbon nano-tubes (MCNTs) find great scope in biomedical, biosensor and biotechnological applications.11–13 MCNTs consist of several graphite layers surrounding a central tubule, while a SCNTs have a central tubule without a graphitic layer.13,15,16 MCNTs are 1-D-nano-particles having numerous features such as higher surface area, more dispersibility, more physico-chemical stability, lower cost and lesser cyto-toxicity compared to SCNTs.11–13,15,16 Till date, very few attempts have been made by various researcher to form enzyme nano-bio-conjugates with carbon-based nano-materials, however there potential biocatalytic applications are still unexplored, which invoke researchers to apply bio-nano-conjugates as a biocatalyst for potential biocatalytic applications to synthesize commercially important organic moieties.13,15,17
In present study, we have used these developed enzyme bio-nano-conjugates for synthesis of various acylated products (commercially important esters) which are extensively used in various fields.20–24 The survey of chemical reactions used in preparation of drug candidate molecule showed that 12% reaction involves the acylation steps.22 Thus acylation is one of the most important transformations, but chemical way of acylation is atom inefficient processes which have several disadvantages such as bi-product formation, use of higher temperature, lower yield-higher waste, higher activation energy and use of hazardous acids or bases.22 Hence, development of greener and waste minimizing biocatalytic methods of acylation will considerably improve the environmental performance for sustainability.22 Recently, the American Chemical Society Green Chemistry Institute (ACS-GCIPR) was established to promote innovations for synthesis of valuable organic moieties using greener chemistry and sustainable technology.23 Acylated moieties are the essential components of the flora, fruity, grasses, essential oils and vegetations etc. which having pleasant fruity smell and taste.24 Moreover, these compounds are widely used in various pharmaceuticals, decorative cosmetics, balm, body lotions, ointment, face cream, fragrance, flavours, shampoos, soaps, shower-shaving gels and other toiletries.24 Most of these acetates are produced in metric of tons per year; furthermore these acetate esters are listed as granted-A substances by the Council of Europe for their safe use in food-stuffs.25 According to BCC research survey, the worldwide market for fragrance compounds was estimated to US $ 11.2 bn in 2011 and projected to raise US $ 15.7 bn upto 2017; while global market for flavour was accounted to US $ 11.3 bn in 2012 and projected almost US $ 14.5 bn up to 2017.26
Thus, considering such a wide range of applications and scope; industries are looking to produce these flavour fragrance compounds by an eco-friendly way so that, they could labelled them as “Safe and Green” products.24,26 Hence in present study, we make an attempt to develop nano-bio-conjugates as an efficient immobilized biocatalyst which was employed for the greener synthesis of various twenty-two food flavour and fragrance compounds along with their commercial importance.
2. Material and methods
2.1 Enzymes and chemicals
Lipase PFL (Pseudomonas fluorescens, activity ≥20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1), lipase HPL (Horse pancreatic lipase, ≥10
000 U g−1), lipase HPL (Horse pancreatic lipase, ≥10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1) p-nitro phenyl butyrate (p-PNB), MCNTs were purchased from Sigma-Aldrich Pvt. Ltd India. Lipase MJL (Mucor javanicus, activity ≥10
000 U g−1) p-nitro phenyl butyrate (p-PNB), MCNTs were purchased from Sigma-Aldrich Pvt. Ltd India. Lipase MJL (Mucor javanicus, activity ≥10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1) was kindly gifted by Amano Enzymes (Japan). All other solvents/chemicals were bought from the Sigma-Aldrich, Alfa Aesar and Hi-media Pvt. Ltd India.
000 U g−1) was kindly gifted by Amano Enzymes (Japan). All other solvents/chemicals were bought from the Sigma-Aldrich, Alfa Aesar and Hi-media Pvt. Ltd India.
2.2 Immobilization of the lipase
200 mg of MCNTs were dispersed by sonication almost around 30–40 minutes in 50 mL of deionised water. Later on, lipase PFL solution (100 mg dissolved in 6–8 mL) was added into above dispersed MCNT solution. The resultant solution was also sonicated with 50% duty cycle (5 minutes ON/OFF mode, with frequency 30 kHz and power 100 W) for 20 minutes. Finally this solution was kept in an orbital shaker for 4–6 hours. Afterwards, the solution mixtures were vacuum-filtrated to get immobilized enzyme and the supernatant was removed. The immobilized enzymes (nano-bio-conjugates denoted as MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL) were then washed two times with 50 mL of deionised water.
PFL) were then washed two times with 50 mL of deionised water.
2.3 Characterization of MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) PFL lipase
PFL lipase
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase) in 1 mL of n-hexane. The reaction was initiated by addition of 1 mL of 12 mM, p-NPB substrate dissolved in iso-propanol solution and incubated at 37 °C for 10 minutes. Afterwards, 200 μL of reaction mixture was withdrawn and added to 600 μL of deionized water to extract p-nitro phenol (p-NP) in the aqueous phase. Finally, 1200 μL of potassium phosphate buffer solution of the pH 7.9–8.0 was added to above mixture in order to give a pale yellow colour for extracted p-NP. This pale yellow coloured sample was instantly used to determine the absorbance at 410 nm. The lipase activity was defined as micro-moles of p-NP released by per milligram of the lipase per minute under the given standard hydrolytic assay condition.
lipase) in 1 mL of n-hexane. The reaction was initiated by addition of 1 mL of 12 mM, p-NPB substrate dissolved in iso-propanol solution and incubated at 37 °C for 10 minutes. Afterwards, 200 μL of reaction mixture was withdrawn and added to 600 μL of deionized water to extract p-nitro phenol (p-NP) in the aqueous phase. Finally, 1200 μL of potassium phosphate buffer solution of the pH 7.9–8.0 was added to above mixture in order to give a pale yellow colour for extracted p-NP. This pale yellow coloured sample was instantly used to determine the absorbance at 410 nm. The lipase activity was defined as micro-moles of p-NP released by per milligram of the lipase per minute under the given standard hydrolytic assay condition.The protein content or protein binding yield or adsorption efficiency for the MCNT immobilized lipase was determined by Bradford methodology at 595 nm.28 The amount of adsorbed protein (lipase) is the difference between amount of protein introduced for immobilization and the amount of protein found in the filtrate/decant after immobilization on the MCNT.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase and native lipase.
lipase and native lipase.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase and free lipase PFL.
lipase and free lipase PFL.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase sample.
lipase sample.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase were determined by the Lineweaver–Burk plot using hydrolytic lipase activity assay of p-NPB in 3–24 mM substrate concentration. The procedure used for Km and Vmax determination is same as indicated in Section 2.3.1.
PFL lipase were determined by the Lineweaver–Burk plot using hydrolytic lipase activity assay of p-NPB in 3–24 mM substrate concentration. The procedure used for Km and Vmax determination is same as indicated in Section 2.3.1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase. Soon after, the reaction vessel was placed in an orbital shaker at a specified temperature and rotation speed.
lipase. Soon after, the reaction vessel was placed in an orbital shaker at a specified temperature and rotation speed.3. Result and discussion
3.1 Immobilization of lipase on MCNT from various microbial sources and their screening
Various immobilized lipases with the support composition MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase (200 mg
lipase (200 mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 mg) was screened to determine % lipase activity, % protein content, % activity retention and % conversion for the model cinnamyl acetate compound formation (Table 1). The % lipase activity is defined as the observed lipase activity ratio of MCNT
120 mg) was screened to determine % lipase activity, % protein content, % activity retention and % conversion for the model cinnamyl acetate compound formation (Table 1). The % lipase activity is defined as the observed lipase activity ratio of MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase to free lipase activity,29 while trend for the % lipase activity was found to be PFL (65.62%) > MJL (48.06%) > HPL (33.74%). Thus, the hydrolysis is prime function of the lipase which is found to be higher for the lipase PFL for given activity assay condition. Moreover, the % protein adsorption is defined as the amount of protein adsorbed to total amount of protein used for immobilization.29 It was observed that % protein adsorption efficiency was found to be higher for lipase PFL (73.57%) compared to the MJL (60.97%) and HPL (53.71%) lipase. The protein loading was found to be moderate because of the physical immobilization of lipase on MCNT support.30,31 The activity retention is the ratio of specific activity of immobilized lipase to free lipase.29 The lipase PFL showed highest % activity retention (89.10%) as compared to the MJL (78.71%) and HPL (62.81%) lipase. Thus, the lipase activity assay and protein content study indicated successful immobilization of various studied lipases (PFL, MJL and HPL) on MCNTs (Table 1). Among all three screened lipases, the lipase PFL showed the best results for immobilization on MCNT which was used for further characterization and biocatalytic application study.
lipase to free lipase activity,29 while trend for the % lipase activity was found to be PFL (65.62%) > MJL (48.06%) > HPL (33.74%). Thus, the hydrolysis is prime function of the lipase which is found to be higher for the lipase PFL for given activity assay condition. Moreover, the % protein adsorption is defined as the amount of protein adsorbed to total amount of protein used for immobilization.29 It was observed that % protein adsorption efficiency was found to be higher for lipase PFL (73.57%) compared to the MJL (60.97%) and HPL (53.71%) lipase. The protein loading was found to be moderate because of the physical immobilization of lipase on MCNT support.30,31 The activity retention is the ratio of specific activity of immobilized lipase to free lipase.29 The lipase PFL showed highest % activity retention (89.10%) as compared to the MJL (78.71%) and HPL (62.81%) lipase. Thus, the lipase activity assay and protein content study indicated successful immobilization of various studied lipases (PFL, MJL and HPL) on MCNTs (Table 1). Among all three screened lipases, the lipase PFL showed the best results for immobilization on MCNT which was used for further characterization and biocatalytic application study.
| Lipase | MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase lipase |
Hydrolytic lipase activity | Lipase activity (%) | Total amount of protein content | Protein adsorbed (%) | Sp. activity MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase lipase |
Activity retention (%) | % Conversion | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Free | MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase lipase |
Non-adsorbed | Adsorbed | Free | MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase lipase |
||||||
a Lipase activity: U mg−1; protein content: μg mg−1; specific activity: U μg−1; acinnamyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vinyl acetate (2 vinyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 mmol); immobilized biocatalyst MCNT 3.5 mmol); immobilized biocatalyst MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipase (42 mg); temperature (50 °C); % lipase activity = immobilized MCNT lipase (42 mg); temperature (50 °C); % lipase activity = immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase activity/free PFL lipase activity; % protein adsorbed = adsorbed amount of protein/(non-adsorbed + adsorbed amount of protein); specific activity = MCNT PFL lipase activity/free PFL lipase activity; % protein adsorbed = adsorbed amount of protein/(non-adsorbed + adsorbed amount of protein); specific activity = MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase activity/protein adsorbed; % activity retention = specific activity of immobilized lipase/specific activity of free lipase. PFL lipase activity/protein adsorbed; % activity retention = specific activity of immobilized lipase/specific activity of free lipase. |
|||||||||||
| PFL | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120 |
59.23 | 38.37 | 65.62 | 12.39 | 34.50 | 73.57 | 1.125 | 89.10 | 56 | 99 |
| MJL | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120 |
11.34 | 5.45 | 48.06 | 22.81 | 35.63 | 60.97 | 0.153 | 78.71 | 11 | 20 |
| HPL | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120 |
7.23 | 2.44 | 33.74 | 45.97 | 53.36 | 53.71 | 0.045 | 62.81 | 13 | 19 |
3.2 Influence of the PFL lipase loading on MCNT
Influence of PFL lipase loading (immobilized lipase PFL compositions MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL) was tested to achieve higher protein adsorption and higher lipase activity. The % lipase activity, % protein adsorption and specific activity of immobilized lipase was increased with increase in lipase loading from 50 to 100 mg per 200 mg of MCNT. This increase in lipase activity might be attributed to availability of higher catalytic sites with higher lipase loading which leads to extend its fullest activity.16,31 However, further increase in lipase loading from 100 to 120 mg per 200 mg of MCNT showed decrease in the % lipase activity, % protein adsorption and specific activity. Since, higher lipase loading may causes increase in stacking of lipase bio-molecules on the support MCNT which restricts the possible mass transfer diffusion of substrates towards the active sites of lipase.30,31 Moreover, heavy loading of lipases resulted into junk adsorption of enzyme molecules on top of those previously immobilized lipases which tends to causes jamming of active sites.13,29 Thus, the optimized quantity of the lipase PFL loading was 100 mg per 200 mg of the MCNT, wherein we obtained highest lipase activity, protein adsorption and specific activity. Similar type of lower lipase activity at higher lipase loading was obtained to Pujari et al.29 for lipase immobilization on polypropylene biphasic membrane (Table 2).
PFL) was tested to achieve higher protein adsorption and higher lipase activity. The % lipase activity, % protein adsorption and specific activity of immobilized lipase was increased with increase in lipase loading from 50 to 100 mg per 200 mg of MCNT. This increase in lipase activity might be attributed to availability of higher catalytic sites with higher lipase loading which leads to extend its fullest activity.16,31 However, further increase in lipase loading from 100 to 120 mg per 200 mg of MCNT showed decrease in the % lipase activity, % protein adsorption and specific activity. Since, higher lipase loading may causes increase in stacking of lipase bio-molecules on the support MCNT which restricts the possible mass transfer diffusion of substrates towards the active sites of lipase.30,31 Moreover, heavy loading of lipases resulted into junk adsorption of enzyme molecules on top of those previously immobilized lipases which tends to causes jamming of active sites.13,29 Thus, the optimized quantity of the lipase PFL loading was 100 mg per 200 mg of the MCNT, wherein we obtained highest lipase activity, protein adsorption and specific activity. Similar type of lower lipase activity at higher lipase loading was obtained to Pujari et al.29 for lipase immobilization on polypropylene biphasic membrane (Table 2).
| No. | MNCT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL (mg) PFL (mg) |
Lipase activity MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL PFL |
% Lipase activity | Amount of protein adsorbed | % Protein adsorbed | Specific activity MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL PFL |
% Activity retention |
|---|---|---|---|---|---|---|---|
| 1 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
15.45 | 26.08 | 13.22 | 67.96 | 1.168 | 38.37 |
| 3 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
31.12 | 52.54 | 23.14 | 74.35 | 1.343 | 70.66 |
| 4 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
41.99 | 70.89 | 29.89 | 76.85 | 1.409 | 92.23 |
| 5 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110 |
40.89 | 69.03 | 32.69 | 76.66 | 1.250 | 89.96 |
| 6 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120 |
38.87 | 65.62 | 34.50 | 73.57 | 1.125 | 89.10 |
| 7 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140 |
36.75 | 62.04 | 37.56 | 69.96 | 0.978 | 88.55 |
3.3 Influence of the immobilization time on % protein content, % lipase activity and specific activity
An immobilized lipase preparation is a crucial parameter to obtain an efficient nano-bio-conjugates (biocatalyst) with the best bio-catalytic activity.13,16 Hence, there must be proper interaction between the immobilization support and the lipase which can be achieved by maintaining the sufficient incubation time. Thus, in present study, we have incubated the lipase from 0 to 5 hours with MCNT for immobilization (Fig. 1). The % lipase activity and % protein content was decreased from the supernatants along with the time span, this fact indicating the successful immobilization of lipase on MCNT. Thus, highest % lipase activity and protein adsorption was found to be nearly at 4 hour incubation. In a similar way, the specific activity was found to be higher at 4 h incubation. Further increase of incubation time span (from 4 h to 6 h) did not have significant effect on the activity and protein adsorption. Higher incubation time promotes the proper hydrophobic interaction of the lipase molecules with the support MCNT for physical adsorption.13,29,30 Moreover it was postulated that, pi–pi stacking interaction between the sidewalls of MCNTs and the aromatic rings of amino acids residues could also contributes for the physical adsorption of the lipase molecules.31 Thus, 4 h is the optimized incubation time for the immobilization of the lipase PFL. | ||
| Fig. 1 Influence of the immobilization time on % protein content, % lipase activity and specific activity. | ||
3.4 FT-IR analysis study
The lipase immobilization on the wall of MCNT was verified by FT-IR spectroscopy (please find ESI† for figure). The most intensive and broadened absorption band at 3500 cm−1 corresponds to vibrations of N–H groups associated with the amide bond. The parent amide functionality of enzyme can be assigned by amides I, II, III bands.16,20 In nano-bio-conjugate (MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL), amide I band at 1600–1700 cm−1 is attributed to C
PFL), amide I band at 1600–1700 cm−1 is attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations, amide II band at 1450–1600 cm−1attribute to N–H bending and C–N stretching vibrations, while amide III band at 1300–1450 cm−1 is attributed to, C–C, C–N stretching and N–H bending vibrations.16,20,31 Similar types of bands are attributed in present study for the native/free PFL lipase and immobilized MCNT–PFL lipase. However, MCNT solely made up of carbon and did not provide any significant information except the C–C bond which is parent bond for the organic functionality compounds. Thus, the present FT-IR spectroscopy confirmed presence of the amide functionality and subsequent immobilization of the lipase on the MCNT.31 Similar type of amide bond functionality after lipase immobilization was confirmed by Gupta et al.,16 and Pavlidis et al.31
O stretching vibrations, amide II band at 1450–1600 cm−1attribute to N–H bending and C–N stretching vibrations, while amide III band at 1300–1450 cm−1 is attributed to, C–C, C–N stretching and N–H bending vibrations.16,20,31 Similar types of bands are attributed in present study for the native/free PFL lipase and immobilized MCNT–PFL lipase. However, MCNT solely made up of carbon and did not provide any significant information except the C–C bond which is parent bond for the organic functionality compounds. Thus, the present FT-IR spectroscopy confirmed presence of the amide functionality and subsequent immobilization of the lipase on the MCNT.31 Similar type of amide bond functionality after lipase immobilization was confirmed by Gupta et al.,16 and Pavlidis et al.31
3.5 SEM and TEM analysis study
The SEM analysis clearly showed distinction in the MCNT and immobilized MCNT since, immobilization of the lipase was clearly observed on the wall of MCNT as shown in Fig. 2. SEM analysis (Fig. 2A and B) showed the surface texture which indicating the change in the surface morphology of MCNT after adsorption of lipase PFL. Moreover, TEM analysis of support MCNT showed presence of clear picture (Fig. 2C) while immobilized sample showed presence of black colour globular spots (Fig. 2D) which indicated adsorption of lipase on MCNT. Similar type of change in textural study was observed to various researchers15,16,19,20 after immobilization of lipase. | ||
Fig. 2 (A) SEM analysis of control MCNT (B) SEM analysis of immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL (C) TEM analysis of control MCNT (D) TEM analysis of immobilized MCNT PFL (C) TEM analysis of control MCNT (D) TEM analysis of immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL. PFL. | ||
3.6 % Water content analysis study
The catalytic and synthetic activity of lipases is well dependent on the degree of hydration which is controlled by trace amount of tightly bound water.1,32,33 Since, minimum amount of bound water is essentially needed to uphold the optimal conformation of the lipase which involves opening and closing movement of lipase lids.3,7,9 Moreover, the bacterial lipase needed higher amount of water content since bacterial lipases such as Pseudomonas fluorescens (PFL) involves large internal movement of lids because of more protein residues.33 Thus to carry out an efficient bio-catalysis there is need of the essential trace amount of the water layer around enzyme. In present study, we have used water as a medium for the immobilization and hence water content for MCNT immobilized lipase was found to be higher (7.8%) as compared to that of the free PFL (1.23%) (Table 3). Thus, it is expected that higher % water content could assist to (i) maintain the conformational changes of three dimensional structures of the enzymes as well as (ii) to improve the catalytic activity and conversion of the desired product.33| No. | Sample | Water content |
|---|---|---|
| 1 | MCNT only | 2.2% |
| 2 | Free PFL (lipase) | 1.23% |
| 3 | Immobilized lipase MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL (2 PFL (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
7.8% |
3.7 Kinetic analysis study (Km, Vmax, and catalytic efficiency)
The Lineweaver–Burk plot for kinetic study of hydrolytic activity assay was used to deduce Km, Vmax and catalytic efficiency (Table 4).1 Interestingly in present study, the Vmax value for free lipase PFL showed slightly higher velocity (67.11) as compared to MCNT immobilized lipase (58.14). In context to this Km value is significantly decreased after the immobilization, this may be attributed due to the immobilization of the lipase which may facilitate the easy enzyme–substrate interaction.21 Thus, lower Km value indicated higher substrate affinity of the immobilized lipase PFL.1,21 In enzyme biochemistry, the presence of uncompetitive inhibition results in decrease of Km and Vmax value after immobilization.1 In uncompetitive inhibition substrate affinity increased while maximum velocity decreased due to pseudo-interaction of enzyme–substrate.1 However overall, catalytic efficiency (Vmax/Km) was found to be higher for the immobilized lipase as compared to the free lipase. Thus the % catalytic efficiency showed 12% higher hydrolytic activity of the MCNT immobilized lipase.1,21,34 The higher catalytic efficiency was attributed due to immobilization of lipase PFL on the hydrophobic surface of MCNT. Thus, lipase immobilization on the hydrophobic surfaces can provided activation of lipases (interfacial activation) which leads to improve catalytic activity of nano-bio-conjugates after lipase immobilization as compared to free lipase PFL.7,9,16,34| No. | Sample | % Water content | Vmax (μmol mg−1 min−1) | Km (mM) | Catalytic efficiency | % Catalytic efficiency | R2 |
|---|---|---|---|---|---|---|---|
| 1 | Free lipase PFL | 1.23 | 67.11 | 8.83 | 7.59 | 100 | 0.978 |
| 2 | Immobilized lipase MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL (2 PFL (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
7.8 | 58.14 | 6.89 | 8.501 | 111.94 | 0.992 |
| 3 | CNT only | 2.2 | — | — | — | — |
3.8 Screening of various immobilized lipase support composition for biocatalytic applications
Initially we have screened various lipase from various microbial sources as biocatalysts (free and MCNT immobilized lipase) for the given reaction system (Scheme 1) (Table 1, last columns). For biocatalytic applications, it was observed that lipase PFL is the best suitable biocatalyst as compared to the lipase MJL and HPL, thus immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase was selected for further study. However, it is necessary to screen immobilization composition of MCNT
PFL lipase was selected for further study. However, it is necessary to screen immobilization composition of MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase to find out the suitable robust biocatalyst composition.13,16,31 Hence, we have varied lipase PFL immobilization quantity by keeping constant amount of the immobilization support (200 mg). It was observed that, as the loading of lipase PFL increases (from 50 to 100) then % conversion also increased which may be attributed due to availability of extra active sites for catalytic transformations (Table 5, entries 1–4). Moreover, further increase in the lipase PFL loading from (100–120 mg) did not have significant effect on the catalytic transformation (Table 5, entries 4–6). Whereas, increase in the lipase loading from 120 to 150 mg causes slight decrease in the bio-catalytic activity because of the crowding or stacking of lipase bio-molecules which may causes blocking of the active catalytic sites of lipase PFL (Table 5, entries 6–8).13,16,31 Similar type of lower enzyme activity was noted by Pavlidis et al.,31 for higher enzyme loading on functionalized carbon-based nonmaterial. Thus, enzyme to nano-material composition is an essential parameter that could affect the catalytic behaviour of immobilized enzymes.
PFL lipase to find out the suitable robust biocatalyst composition.13,16,31 Hence, we have varied lipase PFL immobilization quantity by keeping constant amount of the immobilization support (200 mg). It was observed that, as the loading of lipase PFL increases (from 50 to 100) then % conversion also increased which may be attributed due to availability of extra active sites for catalytic transformations (Table 5, entries 1–4). Moreover, further increase in the lipase PFL loading from (100–120 mg) did not have significant effect on the catalytic transformation (Table 5, entries 4–6). Whereas, increase in the lipase loading from 120 to 150 mg causes slight decrease in the bio-catalytic activity because of the crowding or stacking of lipase bio-molecules which may causes blocking of the active catalytic sites of lipase PFL (Table 5, entries 6–8).13,16,31 Similar type of lower enzyme activity was noted by Pavlidis et al.,31 for higher enzyme loading on functionalized carbon-based nonmaterial. Thus, enzyme to nano-material composition is an essential parameter that could affect the catalytic behaviour of immobilized enzymes.
| No. | Immobilized MNCT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL (mg) PFL (mg) |
Immobilized composition MNCT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL PFL |
% Conversion | Improved catalytic activity in folds | |
|---|---|---|---|---|---|
Immobilized CNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL PFL |
Free lipase PFL | ||||
a Cinnamyl alcohol (2 mmol); vinyl acetate (3.5 mmol); orbital rotation speed (200 rpm); immobilized biocatalyst MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL (42 mg); temperature (50 °C). PFL (42 mg); temperature (50 °C). |
|||||
| 1 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0 | 0 | 0 | 0 |
| 2 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
48 | 22 | 2.18 |
| 3 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
79 | 38 | 2.07 |
| 4 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 | 52 | 1.92 |
| 5 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
99 | 54 | 1.83 |
| 6 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
99 | 56 | 1.76 |
| 7 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
97 | 58 | 1.67 |
| 8 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
96 | 61 | 1.58 |
3.9 Optimization of developed biocatalytic protocol
To achieve maximum conversion of desired product, molar ratio of cinnamyl alcohol to vinyl acetate was varied from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 to 2
0.5 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (Table 6, entries 1–8). Experimentally, it was observed that increase of moles of vinyl acetate from 0.5 to 3.5 led to increase in the % conversion of the desired product when reaction catalyzed by immobilized lipase. However, further increase of moles of vinyl acetate from 3.5 to 4 did not offer any significant increment in the conversion of desired product. In context to this, % conversion was increased with increase in moles of vinyl acetate from 0.5 to 4 mmol when reaction is catalyzed by free lipase PFL (conversion showed in parentheses, Table 6, last column). When vinyl acetate used as an acyl donor then vinyl alcohol was formed as a by-product which is an unstable species, tautomerizes into acetaldehyde and did not take part in subsequent reaction.4 Thus, 2
4 (Table 6, entries 1–8). Experimentally, it was observed that increase of moles of vinyl acetate from 0.5 to 3.5 led to increase in the % conversion of the desired product when reaction catalyzed by immobilized lipase. However, further increase of moles of vinyl acetate from 3.5 to 4 did not offer any significant increment in the conversion of desired product. In context to this, % conversion was increased with increase in moles of vinyl acetate from 0.5 to 4 mmol when reaction is catalyzed by free lipase PFL (conversion showed in parentheses, Table 6, last column). When vinyl acetate used as an acyl donor then vinyl alcohol was formed as a by-product which is an unstable species, tautomerizes into acetaldehyde and did not take part in subsequent reaction.4 Thus, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 was optimized mole ratio quantity providing 98% conversion of desired product and was chosen to carry out remaining all experiments.
3.5 was optimized mole ratio quantity providing 98% conversion of desired product and was chosen to carry out remaining all experiments.
| No. | Solvent | Alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acyl donor (mmol) acyl donor (mmol) |
Rotation (rpm) | Biocatalyst (mg) | Temperature (°C) | Conversiona (%) |
|---|---|---|---|---|---|---|
a Conversion indicated in parenthesis is obtained by free lipase PFL, while % conversion indicated outside of parenthesis is obtained by immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase (nano-bio-conjugates). PFL lipase (nano-bio-conjugates). |
||||||
| Effect of mole ratio | ||||||
| 1 | Toluene | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
200 | 36 | 50 | 9 (0) |
| 2 | Toluene | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
200 | 36 | 50 | 24 (5) |
| 3 | Toluene | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
200 | 36 | 50 | 40 (10) |
| 4 | Toluene | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
200 | 36 | 50 | 53 (18) |
| 5 | Toluene | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
200 | 36 | 50 | 72 (25) |
| 6 | Toluene | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
200 | 36 | 50 | 85 (36) |
| 7 | Toluene | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
200 | 36 | 50 | 98 (45) |
| 8 | Toluene | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
200 | 36 | 50 | 98 (55) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Effect of solvent | ||||||
| 9 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
200 | 36 | 50 | 99 (46) |
| 10 | Toluene | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
200 | 36 | 50 | 98 (45) |
| 11 | 1,4 dioxane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
200 | 36 | 50 | 82 (26) |
| 12 | Diethyl ether | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
200 | 36 | 50 | 78 (22) |
| 13 | Acetone | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
200 | 36 | 50 | 76 (20) |
| 14 | Acetonitrile | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
200 | 36 | 50 | 28 (4) |
| 15 | Chloroform | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
200 | 36 | 50 | 64 (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Effect of rotation | ||||||
| 16 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
0 | 36 | 50 | 21 (06) |
| 17 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
50 | 36 | 50 | 48 (20) |
| 18 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
100 | 36 | 50 | 68 (30) |
| 19 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
150 | 36 | 50 | 93 (41) |
| 20 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 36 | 50 | 99 (48) |
| 21 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
200 | 36 | 50 | 99 (46) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Effect of biocatalyst | ||||||
| 22 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 0 | 50 | 0 (0) |
| 23 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 10 | 50 | 40 (14) |
| 24 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 20 | 50 | 68 (27) |
| 25 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 30 | 50 | 90 (45) |
| 26 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 36 | 50 | 99 (48) |
| 27 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 40 | 50 | 99 (50) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Effect of temperature | ||||||
| 28 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 36 | 25 | 21 (5) |
| 29 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 36 | 30 | 38 (11) |
| 30 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 36 | 35 | 54 (22) |
| 31 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 36 | 40 | 68 (35) |
| 32 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 36 | 45 | 85 (47) |
| 33 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 36 | 50 | 99 (48) |
| 34 | n-Heptane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
175 | 36 | 55 | 99 (48) |
To find out suitable reaction media, we have tested various polar and non-polar organic solvents having log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ρ value in the range of 0.4 to 4.0 for the given model reaction. In present study, the polar solvents such as 1,4 dioxane, diethyl ether, acetone and chlorinated solvent such as chloroform gave moderate to good conversion of corresponding product while, non-polar solvents like toluene and n-heptane were found to be promising solvent to offer excellent conversion of the desired product (Table 6, entries 9–15). This was anticipated due to better hold up of essential bound water from enzyme coat in presence of non-polar solvent which avoids distortion of lipase conformations and hence offered best catalytic efficiency.1–3 Since, polar solvent causes distortion of enzyme confirmation and subsequent catalytic activity due to stripping of essential bound water.23,32,33
ρ value in the range of 0.4 to 4.0 for the given model reaction. In present study, the polar solvents such as 1,4 dioxane, diethyl ether, acetone and chlorinated solvent such as chloroform gave moderate to good conversion of corresponding product while, non-polar solvents like toluene and n-heptane were found to be promising solvent to offer excellent conversion of the desired product (Table 6, entries 9–15). This was anticipated due to better hold up of essential bound water from enzyme coat in presence of non-polar solvent which avoids distortion of lipase conformations and hence offered best catalytic efficiency.1–3 Since, polar solvent causes distortion of enzyme confirmation and subsequent catalytic activity due to stripping of essential bound water.23,32,33
Influence of the mass transfer is an important factor to achieve proper substrate and catalyst interaction.8,23 Hence mass transfer effect was studied in the range of the 0 to 200 rpm (Table 6, entries 16–21). It was seen that, % conversion was increased as the rotation speed increases from 0 to 175 rpm. Thus the mass transfer barrier was achieved at the 175 rpm which providing highest % conversion. Higher rotation (>230 rpm) speed leads to decrease in the % conversion, as immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL biocatalyst was thrown outside of reaction media and did not take part in further reaction. Hence, 175 rpm was the optimized rotation speed for the given reaction system.
PFL biocatalyst was thrown outside of reaction media and did not take part in further reaction. Hence, 175 rpm was the optimized rotation speed for the given reaction system.
In order to maintain an economic feasibility, we studied effect of the biocatalyst amount to find out optimum concentration of biocatalyst.16,18 The immobilized lipases were loaded ranging from 0–40 mg while free/crude lipase were also loaded in w/w equivalent amount (Table 6, entries 22–27). It was observed that biocatalyst loading (free as well as immobilized lipase) showed positive relationship with % conversion which was attributed to active participation of the available catalytic sites to carry out reaction. The maximum conversion (99%) of cinnamyl acetate was obtained when reaction was catalyzed by 36 mg of immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase. In contrast to this equivalent quantity of free lipase PFL provided almost 2 fold lesser conversion. Further, increase in the biocatalyst amount to 40 mg did not afford significant improvement in the % conversion.
PFL lipase. In contrast to this equivalent quantity of free lipase PFL provided almost 2 fold lesser conversion. Further, increase in the biocatalyst amount to 40 mg did not afford significant improvement in the % conversion.
It is well known fact that, enzymes are usually active at optimum temperatures whereas their catalytic activity–stability goes on decreasing beyond the optimum temperature due to confirmation destabilization.3,6,9,18 Hence, in current study, the MCNT immobilized lipase was subjected for acetate synthesis at temperature ranging from 25–55 °C (Table 6, entries 28–34). The increase of temperature from 25–50 °C led to increase in % conversion of the desired product and thus 50 °C was observed to be optimum temperature for immobilized lipase, since 55 °C displayed similar % conversion at given reaction condition. The higher energy provided activation energy to the reacting molecules and speed up the reaction rate. These results were signifying that, 50 °C was chosen as optimized temperature and used for further all experiments. Thus the optimized reaction parameters are: reactant molar ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5), biocatalyst amount (36 mg), rotation speed (175 rpm) and temperature (50 °C).
3.5), biocatalyst amount (36 mg), rotation speed (175 rpm) and temperature (50 °C).
3.10 Application of MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) PFL nano-bio-conjugates for syntheses of acylated moieties and their potential applications
PFL nano-bio-conjugates for syntheses of acylated moieties and their potential applications
The potential biocatalytic applications of lipase nano-bio-conjugates were tested for synthesis of various important organic moieties by using optimized reaction parameters. Enzymatically synthesized compounds are listed in Table 7, which possessing the great importance and application as a food, flavour/fragrance compounds in various commercial fields.24,25,35–39 Most of these compounds have natural occurrence and can be obtained by tedious costly natural extraction process. In context to this, enzymatic synthesis is more efficient which can labelled these products as “Green and Natural” for safe use in day to day life.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL immobilized lipase for syntheses of various organic compounds and their potential applicationsa
PFL immobilized lipase for syntheses of various organic compounds and their potential applicationsa
| No. | Flavour–fragrance compound | Time (h) | % Conversion | Application in food flavour–fragrance | Natural occurrence in plants and fruits | |
|---|---|---|---|---|---|---|
Immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL PFL |
Free PFL | |||||
a Alcohol (2 mmol); vinyl acetate (3.5 mmol); orbital rotation speed (175 rpm); immobilized biocatalyst MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL (36 mg); temperature (50 °C). PFL (36 mg); temperature (50 °C). |
||||||
| 1 | 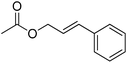 |
4 | 99 | 48 | Cinnamon, oriental, rose, apricot, guava | Psidium guajava, Laurus nobilis, Cinnamomum verum |
| 2 |  |
4 | 99 | 42 | Pear, banana, lavender, melon, pineapple, peach | Cananga odorata |
| 3 |  |
4.5 | 99 | 36 | Strawberry, tomato, cherry, melon, plum, | Averrhoa carambola, Theobroma cacao |
| 4 | 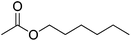 |
5 | 99 | 38 | Apple, banana, pear, mulberry, papaya, mango, plum | Citrus bergamia, Michelia champaca, Fragaria vesca |
| 5 | 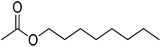 |
5 | 99 | 37 | Tea green peach, apple, gardenia, | Boswellia carterii, Lavandula angustifolia |
| 6 | 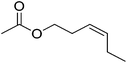 |
5 | 99 | 41 | Quince, orchid, fig, gooseberry, jackfruit, melon | Jasminum flexile, Vaccinium myrtillus, matricaria sp. |
| 7 | 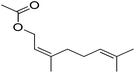 |
5.5 | 99 | 41 | Peach, lemon, blackberry, Papaya, lavender | Citrus bergamia, Elettaria cardamomum |
| 8 | 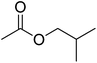 |
4.5 | 99 | 38 | Pineapple, current, butter, raspberry | Theobroma cacao, Fragaria vesca |
| 9 | 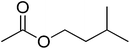 |
4 | 99 | 39 | Vanilla, plum, peach, date | Theobroma cacao, Musa sapientum |
| 10 | 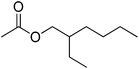 |
5 | 99 | 38 | Pea, bean, fungus mushroom, oriental | — |
| 11 | 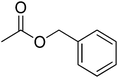 |
4 | 99 | 48 | Jasmine, green apple, strawberry, banana, orchid, | Acacia farnesiana, Hyacinthus sp. Jasminum |
| 12 | 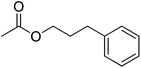 |
4 | 99 | 46 | Blackberry, cinnamon, lily, cranberry | Llaurus nobilis, Narcissus spp., Psidium guajava |
| 13 | 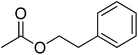 |
4 | 99 | 47 | Apricot, peach vanilla, tutti-frutti | Freesia magnolia, Hyacinth reseda |
| 14 | 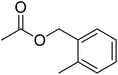 |
5 | 99 | 30 | — | Cyclamen persicum, Cyclamen purpurescens |
| 15 |  |
4.5 | 99 | 39 | Juniper berry, raspberry, vanilla, fig, coconut cherry | Cananga odorata, Clavija euerganea, Jacquinia keyensis |
| 16 | 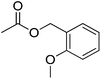 |
5.5 | 99 | 29 | Bayberry, fig, oriental, vanilla | — |
| 17 | 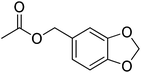 |
4.5 | 99 | 39 | Apricot, peach, raspberry, woody, cherry strawberry | Cichorium endivia |
| 18 | 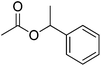 |
12 | 46 | 17 | Sweet pea, pear, apple, grape, plum, berry | Barosma betulina, Eucalyptus globules, Mentha piperita |
| 19 | 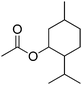 |
12 | 44 | 15 | Peppermint, mint, blueberry, citrus, lavender, peach | Barosma betulina, Eucalyptus globules, Mentha piperita |
| 20 | 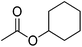 |
12 | 57 | 20 | Apple, pear, currant, fig, jackfruit | Sauerkraut (Sour cabbage) |
| 21 | 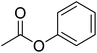 |
12 | 63 | 23 | — | Silene italica, Silene vulgaris |
| 22 | 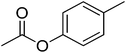 |
12 | 69 | 29 | Apple blossom, cherry blossom | Prangos uechtritzii, Cananga odorata |
The allylic alcohols such as cinnamyl alcohol and prenol (possessing floral-rose soapy smell) (Table 7, entries 1 and 2) gave 99% conversion of corresponding product in 4 h. Acetates of the saturated, linear and branched primary alcohols (having sweet fruity olfaction) also offered excellent yield up to 99% of respective desired product (Table 7, entries 3–10) in 4–6 h. Aromatic side-chain alcohols as like benzyl alcohol, 2-phenyl ethanol and 3-phenyl propanol (possessing fruity-flavour and fragrance) gave excellent yield (99%) of the desired aromatic acetate product in 4 h (Table 7, entries 11–13). Various acetates of substituted benzyl alcohols (Table 7, entries 14–17) were synthesized efficiently with 99% yield within 4–5 h. α-Methyl benzyl acetate (Table 7, entry 18) gave 46% yield of desired product in 12 h while alicyclic secondary acetates of menthol as well as cyclohexanol (Table 7, entry 19 and 20) were synthesized with 44 and 57% yield in 12 h respectively. Phenol and p-cresol (Table 7, entry 21 and 22) were reacted slowly because of acidic nature to provide moderate yield (63 and 69% respectively) of desired acetates in 10 h.
Many of these above organic moieties are of great importance in day to day life compounds24,25 (Table 7, entries 1–22) which can be synthesized enzymatically and are recognized as safe compounds by Council of Europe25 and Flavour Manufacturers.35 In addition to this, these synthesized compounds are approved by the Food Drug Administration (FDA)36 and the Joint Expert Committee on Food Additives (JECFA)37 for safe use as an additives in foods. Thus, many of the above synthesized compounds were also used essentially as an ingredient for the various perfumery and fragrances compounds (Table 7, entries 1–22).36,37 The universal production and use of each flavour or fragrance compound is in the range of 1 to 1000 metric tons per annum.38 The environmental legislation, competitiveness and social responsibility always directing several industries towards development of more ecofriendly, safer and greener industrial commercial processes for the sustainable future.39 Thus looking to the above importance, aspects and wide substrate array, the present protocol demonstrated a promising alternative to synthesize commercially important organic moieties with greener outlook for sustainable future.
3.11 Time versus conversion plot and comparison of bio-catalytic activity between immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) PFL and free PFL
PFL and free PFL
The Fig. 3 depicted the profile of the product formation by free and immobilized CNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase with respect to reaction time progress. The profile of desired product formation with respect to time progress demonstrated improved biocatalytic activity of the immobilized MCNT
PFL lipase with respect to reaction time progress. The profile of desired product formation with respect to time progress demonstrated improved biocatalytic activity of the immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase compared to the corresponding free lipase PFL. The highest conversion of cinnamyl acetate 99% was achieved in 240 minutes (4 h) when reaction was catalyzed by immobilized MCNT
PFL lipase compared to the corresponding free lipase PFL. The highest conversion of cinnamyl acetate 99% was achieved in 240 minutes (4 h) when reaction was catalyzed by immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase, whereas free PFL provided almost 2 fold lesser conversion of the desired product. The continuous increase in the % conversion representing that, reaction was progressed towards forward direction only to offer desired stable product. The improved conversion of the immobilized MCNT
PFL lipase, whereas free PFL provided almost 2 fold lesser conversion of the desired product. The continuous increase in the % conversion representing that, reaction was progressed towards forward direction only to offer desired stable product. The improved conversion of the immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase was attributed to (i) interfacial activation of lipase PFL after immobilization on hydrophobic MCNT7,9 (ii) more catalytic sites are easily accessible after immobilization of lipase PFL on MCNT, which facilities the easy substrate binding to lift-up reaction rate31 and (iii) lipases PFL are well scatters after immobilization on MCNT which favours easy mass transfer of the reactant/product.7,9,31
PFL lipase was attributed to (i) interfacial activation of lipase PFL after immobilization on hydrophobic MCNT7,9 (ii) more catalytic sites are easily accessible after immobilization of lipase PFL on MCNT, which facilities the easy substrate binding to lift-up reaction rate31 and (iii) lipases PFL are well scatters after immobilization on MCNT which favours easy mass transfer of the reactant/product.7,9,31
 | ||
Fig. 3 Product formation profile and comparison of immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL with free PFL for biocatalytic application. PFL with free PFL for biocatalytic application. | ||
3.12 Mechanism of MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) PFL lipase catalyzed cinnamyl acetate synthesis
PFL lipase catalyzed cinnamyl acetate synthesis
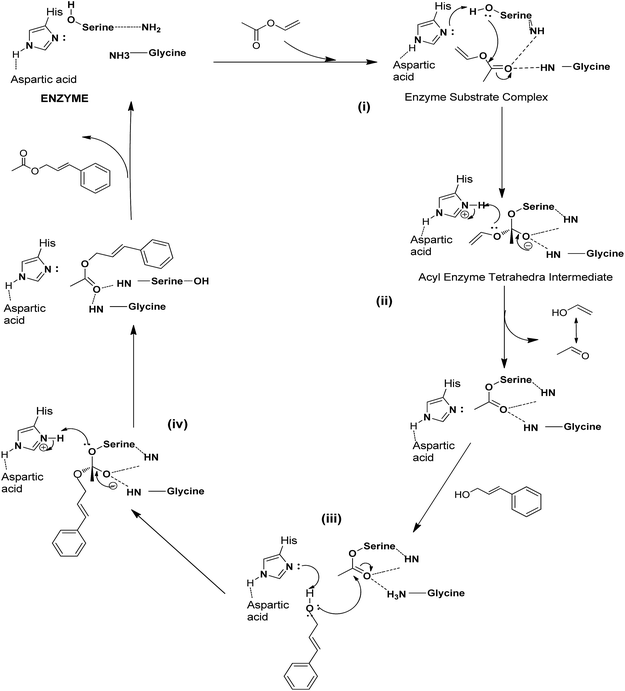
The active centre present in enzyme lipase is known as the catalytic triad which is mainly consist of the serine, aspartic acid and histidine residue, these three residues plays an important role to perform the bio-catalytic activity.40 (i) Initially nano-bio-conjugate MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL forms an enzyme substrate complex along with the acyl donor vinyl acetate (Scheme 2). (ii) Afterwards, the serine group attacks on the electron deficient carbonyl part of the vinyl acetate and tend to form the acyl-lipase intermediate. This acyl enzyme intermediate causes elimination of the bi-product vinyl alcohol which tautomerized into a low boiling acetaldehyde compounds (21 °C). (iii) Soon after, cinnamyl alcohol incorporates in the reaction and attacks on the electron deficient carbonyl group and again forms a tetravalent intermediate with lipase. (iv) This tetravalent intermediate reorganized itself and breaks down into the corresponding desired acetate product and leads to free up lipase molecules (Scheme 2). During overall biocatalytic process, activation of the histidine residue was carried out by the aspartic acid which imparts in the abstraction of the proton. Whereas negative charge developed on the each tetravalent intermediate is significant for the formation of respective product and bi-product. Moreover, various amino-acid residues are responsible for the formation of H-bonding which induces the sufficient polarisation in a molecule so that electrophilic species can easily attacked by nucleophilic species.40
PFL forms an enzyme substrate complex along with the acyl donor vinyl acetate (Scheme 2). (ii) Afterwards, the serine group attacks on the electron deficient carbonyl part of the vinyl acetate and tend to form the acyl-lipase intermediate. This acyl enzyme intermediate causes elimination of the bi-product vinyl alcohol which tautomerized into a low boiling acetaldehyde compounds (21 °C). (iii) Soon after, cinnamyl alcohol incorporates in the reaction and attacks on the electron deficient carbonyl group and again forms a tetravalent intermediate with lipase. (iv) This tetravalent intermediate reorganized itself and breaks down into the corresponding desired acetate product and leads to free up lipase molecules (Scheme 2). During overall biocatalytic process, activation of the histidine residue was carried out by the aspartic acid which imparts in the abstraction of the proton. Whereas negative charge developed on the each tetravalent intermediate is significant for the formation of respective product and bi-product. Moreover, various amino-acid residues are responsible for the formation of H-bonding which induces the sufficient polarisation in a molecule so that electrophilic species can easily attacked by nucleophilic species.40
3.13 Thermo-kinetic investigation of the present biocatalytic protocol
Arrhenius equation is useful to deduce the kinetic parameters such as energy of activation while, Eyring equation is useful to deduce the various thermodynamic parameters such as ΔG*, ΔS*, ΔH*. Herein eqn (1)–(3) involves; k, A, Ea, T, kB, h, R, ΔG*, ΔS*, and ΔH* are reaction rate constant, Arrhenius collision factor, energy of activation, absolute temperature, Boltzmann constant, Planck's constant real gas constant, Gibbs free energy, entropy and enthalpy of activation respectively.
k = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(Ea/RT) exp(Ea/RT)
| (1) |
| ΔG*/T = (ΔH*/T) − ΔS | (2) |
kh = TkB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(ΔS*/R)exp(−ΔH*/RT) exp(ΔS*/R)exp(−ΔH*/RT)
| (3) |
Various thermo kinetic parameters were calculated in the range of range of 303 to 323 K (Table 8). The Ea for free PFL and immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL was found to be 74
PFL was found to be 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149 and 58
149 and 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671 J mol−1 respectively. The lower Ea value for the MCNT immobilized lipase PFL indicated the (i) higher reaction rate (ii) higher catalytic efficacy and (iii) lesser energy requirement as compared to free PFL.41 The lesser enthalpy of activation (ΔH* value) value for MCNT immobilized lipase PFL was attributed due to easy formation of acyl–enzyme complex; while ΔH* was found to be higher for free PFL lipase catalyzed reaction which point to complexity to tetrahedral acyl–enzyme complex formation.42,43 Since, free lipases PFL were agglomerated in organic solvents and created diffusion restrictions for substrate towards active catalytic sites.42–44 The ΔS* value stands for the difference in the extent of local disorder or randomness between ground state and transition state.42–45 The free lipase PFL showed higher disorder (−106.83 J mol−1 K−1), while immobilized MCNT
671 J mol−1 respectively. The lower Ea value for the MCNT immobilized lipase PFL indicated the (i) higher reaction rate (ii) higher catalytic efficacy and (iii) lesser energy requirement as compared to free PFL.41 The lesser enthalpy of activation (ΔH* value) value for MCNT immobilized lipase PFL was attributed due to easy formation of acyl–enzyme complex; while ΔH* was found to be higher for free PFL lipase catalyzed reaction which point to complexity to tetrahedral acyl–enzyme complex formation.42,43 Since, free lipases PFL were agglomerated in organic solvents and created diffusion restrictions for substrate towards active catalytic sites.42–44 The ΔS* value stands for the difference in the extent of local disorder or randomness between ground state and transition state.42–45 The free lipase PFL showed higher disorder (−106.83 J mol−1 K−1), while immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase demonstrated lesser disorder (−146.65 J mol−1·K−1) of reaction. Thus, immobilized MCNT
PFL lipase demonstrated lesser disorder (−146.65 J mol−1·K−1) of reaction. Thus, immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL favoured the reaction with lesser extent of disorder compared to crude lipase PFL. The feasibility of reaction system is best accounted by ΔG* value, lesser is the Gibb's free energy change; higher is the feasibility of reaction and vice-a-versa. Thus immobilized MCNT
PFL favoured the reaction with lesser extent of disorder compared to crude lipase PFL. The feasibility of reaction system is best accounted by ΔG* value, lesser is the Gibb's free energy change; higher is the feasibility of reaction and vice-a-versa. Thus immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL offered smaller ΔG* value (101.97 J mol−1), which demonstrating the higher feasibility of reaction as compared to free PFL (105.18 J mol−1).42–46 Thus from above all thermodynamic parameters, the immobilized MCNT
PFL offered smaller ΔG* value (101.97 J mol−1), which demonstrating the higher feasibility of reaction as compared to free PFL (105.18 J mol−1).42–46 Thus from above all thermodynamic parameters, the immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL offered ease of reaction system compared to that of free PFL. These results indicating the lower thermodynamic energy assessment and improved catalytic activity of hydrophobic MCNT supported lipase PFL (nano-bio-conjugate).
PFL offered ease of reaction system compared to that of free PFL. These results indicating the lower thermodynamic energy assessment and improved catalytic activity of hydrophobic MCNT supported lipase PFL (nano-bio-conjugate).
| No. | Thermo-kinetic factor | MCNT–PFL | Free PFL |
|---|---|---|---|
| 1 | Ea (J mol−1) | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671.80 671.80 |
74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149.24 149.24 |
| 2 | ΔH* (J mol−1) | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 072.11 072.11 |
71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 549.45 549.45 |
| 3 | ΔS* (J mol−1 K−1) | −146.65 | −106.83 |
| 4 | ΔG* (J mol−1) | 101.97 | 105.18 |
3.14 Recyclability and leaching study
The recyclability of immobilised lipase is a key aspect for potential industrial applications and economical feasibility; hence reusability was studied for given model reaction under identical optimised reaction conditions. After each cycle immobilized lipase was washed/rinsed 2 times by n-heptane to remove any stick of products or reactants. It was then dried for 10–12 hours and stored at 0–5 °C until used for next recycle study. It was observed that, conversion decreases as number of recycles increases, while 63% conversion was achieved at the end of the fifth cycle (including fresh) (Fig. 4A). The decrease in % conversion may attributed by leakage of enzyme from MCNT surface, as lipases are physically adsorbed on the surface of MCNT.18,23,47,48 For the leaching study, we have performed an experiment, wherein lipase was placed in reaction medium n-heptane (without reactants) for 1–10 hours at 175 rpm (Fig. 4B). It was observed that lipase was slowly leached from the surface of MCNT which may be attributed to physical adsorption type of immobilization. Thus the leaching was found to be 6.5% and 9% at interval of 6 and 10 hours. Moreover, the decrease in % conversion is also a cause of handling loss and inhibition by alcoholic substrate (cinnamyl alcohol) which forms a dead-end inhibition complex and reduces the enzyme activity.4,6,184. Conclusions
In present study, we have successfully immobilized lipase PFL on the MCNT, this nano-bio-conjugate worked as robust biocatalyst. The composition MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL (2
PFL (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed highest % lipase activity, % protein binding yield and % activity retention. The immobilization of the lipase PFL on the MCNT was confirmed by physical and biochemical characterization such as SEM, TEM, FTIR, Km, Vmax, catalytic efficiency and (%) water content analysis. This immobilized biocatalyst MCNT
1) showed highest % lipase activity, % protein binding yield and % activity retention. The immobilization of the lipase PFL on the MCNT was confirmed by physical and biochemical characterization such as SEM, TEM, FTIR, Km, Vmax, catalytic efficiency and (%) water content analysis. This immobilized biocatalyst MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL (2
PFL (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was successfully applied for O-acylation reactions as a practical biocatalytic applications. For O-acylation reaction, various five reaction parameters were optimized in details. Furthermore these developed biocatalytic protocol was then successfully extended to synthesize several (twenty-two) industrially important acylated moieties with excellent conversion. These products are well characterized by the available tools such as 1H NMR, 13C NMR and GC-MS analysis. Besides this, we have reviewed the potential commercial applications of the synthesized moieties. Moreover, the thermodynamic study indicating more feasibility of immobilized MCNT
1) was successfully applied for O-acylation reactions as a practical biocatalytic applications. For O-acylation reaction, various five reaction parameters were optimized in details. Furthermore these developed biocatalytic protocol was then successfully extended to synthesize several (twenty-two) industrially important acylated moieties with excellent conversion. These products are well characterized by the available tools such as 1H NMR, 13C NMR and GC-MS analysis. Besides this, we have reviewed the potential commercial applications of the synthesized moieties. Moreover, the thermodynamic study indicating more feasibility of immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase (nano-bio-conjugate) over free lipase PFL. Also, immobilized MCNT
PFL lipase (nano-bio-conjugate) over free lipase PFL. Also, immobilized MCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFL lipase showed 2.2 folds higher catalytic activity and five times recyclability compared to free PFL. Thus present protocol is a robust, efficient and recyclable which carry out the (i) synthesis of nano-bio-conjugate as a bio-catalyst, (ii) detail physical–biochemical characterization of biocatalyst, (iii) optimization of biocatalytic protocol (iv) practical biocatalytic applications along with mechanistic study (v) thermodynamic feasibility study and (vi) recyclability study.
PFL lipase showed 2.2 folds higher catalytic activity and five times recyclability compared to free PFL. Thus present protocol is a robust, efficient and recyclable which carry out the (i) synthesis of nano-bio-conjugate as a bio-catalyst, (ii) detail physical–biochemical characterization of biocatalyst, (iii) optimization of biocatalytic protocol (iv) practical biocatalytic applications along with mechanistic study (v) thermodynamic feasibility study and (vi) recyclability study.
Acknowledgements
The author Kirtikumar Badgujar is grateful to the Department of Biotechnology-DBT, India (BT/PR4192/PID/6/623/2011), and the Council of Scientific & Industrial Research-CSIR, India (09/991(0015)/2011-EMR-I), for providing research funds. Also author is greatly thankful to Ms Poorna Pai and Mr Rajesh Patil for their kind help in characterization/analysis.Notes and references
- D. L. Nelson and M. M. Cox, Lehninger's Principles of Biochemistry, W. H. Freeman, New York, 5th edn, 2008 Search PubMed
.
- J. Ge, D. Lu, J. Wang, M. Yan, Y. Lu and Z. Liu, J. Phys. Chem. B, 2008, 112, 14319–14324 CrossRef CAS PubMed
.
- M. Marciello, M. Filice and J. M. Palomo, Catal. Sci. Technol., 2012, 2, 1531–1543 CAS
.
- K. C. Badgujar and B. M. Bhanage, J. Phys. Chem. B, 2014, 118, 14808–14819 CAS
.
- H. Zhou, L. Yang, L. W. Li, F. Wang, W. Li, J. Zhao, X. Liang and H. Liu, Ind. Eng. Chem. Res., 2012, 51, 13173–13181 CrossRef CAS
.
- C. Garcia, I. I. Junior, R. O. M. A. de Souzab and R. Luque, Catal. Sci. Technol., 2015, 5, 1840–1846 CAS
.
- O. Barbosa, M. Ruiz, C. Ortiz, M. Fernández, R. Torres and R. Fernandez-Lafuente, Process Biochem., 2012, 47, 867–876 CrossRef CAS PubMed
.
- K. C. Badgujar and B. M. Bhanage, Ind. Eng. Chem. Res., 2014, 53, 18806–18815 CrossRef CAS
.
- G. Fernandez-Lorente, Z. Cabrera, C. Godoy, R. Fernandez-Lafuente, J. M. Palomo and J. M. Guisan, Process Biochem., 2008, 43, 1061–1067 CrossRef CAS PubMed
.
- Z. X. Tang, J. Q. Qian and L. E. Shi, Characterizations of immobilized neutral proteinase on chitosan nano-particles, Process Biochem., 2006, 41, 1193–1197 CrossRef CAS PubMed
.
- D. Q. Yang, J. F. Rochette and E. Sacher, J. Phys. Chem. B, 2005, 109, 7788–7794 CrossRef CAS PubMed
.
- M. Patila, I. V. Pavlidis, E. K. Diamanti, P. Katapodis, D. Gournis and H. Stamatis, Process Biochem., 2013, 48, 1010–1017 CrossRef CAS PubMed
.
- S. S. Karajanagi, A. A. Vertegel, R. S. Kane and J. S. Dordick, Langmuir, 2004, 20, 11594–11599 CrossRef CAS PubMed
.
- Y. Z. Chen, C. B. Ching and R. Xu, Process Biochem., 2009, 44, 1245–1251 CrossRef CAS PubMed
.
- H. Tan, W. Feng and P. Ji, Bioresour. Technol., 2012, 115, 172–176 CrossRef CAS PubMed
.
- A. Gupta, S. R. Dhakate, M. Pahwa, S. Sinha, S. Chand and R. Mathur, Process Biochem., 2013, 48, 124–132 CrossRef CAS PubMed
.
- P. Asuri, S. S. Karajanagi, H. Yang, T. J. Yim, R. S. Kane and J. S. Dordick, Biotechnol. Bioeng., 2006, 95, 804–811 CrossRef CAS PubMed
.
- C. Y. Shang, W. X. Li and R. F. Zhang, Enzyme Microb. Technol., 2014, 61–62, 28–34 CrossRef CAS PubMed
.
- L. S. Wan, B. B. Ke and Z. K. Xu, Enzyme Microb. Technol., 2008, 42, 332–339 CrossRef CAS PubMed
.
- K. C. Badgujar and B. M. Bhanage, Enzyme Microb. Technol., 2014, 57, 16–25 CrossRef CAS PubMed
.
- O. Barbosa, C. Ortiz, Á. Berenguer-Murcia, R. Torres, R. C. Rodrigues and R. Fernandez-Lafuente, RSC Adv., 2014, 4, 1583–1600 RSC
.
- J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 CAS
.
- K. C. Badgujar, K. P. Dhake and B. M. Bhanage, Process Biochem., 2013, 48, 1335–1347 CrossRef CAS PubMed
.
- K. Bauer, D. Garbe and H. Surburg, Common fragrance and flavour materials: preparation, properties, and uses, Wiley-Vch, 4th edn, 2001 Search PubMed
.
- Council of Europe, Partial Agreement in the Social and Public Health Field. Chemically-defined Flavouring Substances, Group 9.4.1.2. Esters of Aliphatic Acids, Straight Chain, Saturated Acetates, No. 204, Council of Europe Publishing, Strasbourg, 2000, p. 225 Search PubMed
.
- http://www.bccresearch.com/market-research/chemicals/flavors-fragrances-global-markets-chm034c.html.
- G. Pencreacha and J. Barattia, Comparison of hydrolytic activity in water and heptane for thirty-two commercial lipase preparations, Enzyme Microb. Technol., 2001, 28, 473–479 CrossRef
.
- M. Bradford, Anal. Biochem., 1976, 72, 248 CrossRef CAS
.
- N. S. Pujari, B. K. Vaidya, B. K. Ponrathnam and S. Nene, Poly(urethane methacrylate-co-glycidyl methacrylate)-supported-polypropylene biphasic membrane for lipase immobilization, J. Membr. Sci., 2006, 285, 395–403 CrossRef CAS PubMed
.
- C. Chao, J. Liu, J. Wang, Y. Zhang, B. Zhang, Y. Zhang, X. Xiang and R. Che, ACS Appl. Mater. Interfaces, 2013, 5, 10559–10564 CAS
.
- I. V. Pavlidis, T. V. Vorhaben, T. Tsoufis, P. Rudolf, U. T. Bornscheuer, D. Gournis and H. Stamatis, Bioresour. Technol., 2012, 115, 164–171 CrossRef CAS PubMed
.
- N. A. Turner and E. N. Vulfson, Enzyme Microb. Technol., 2000, 27, 108–113 CrossRef CAS
.
- A. A. Mendes, P. C. Oliveira, A. M. Vélez, R. C. Giordano, R. L. C. Giordano and H. F. de Castro, Int. J. Biol. Macromol., 2012, 50, 503–511 CrossRef CAS PubMed
.
- R. Say, R. Keçili, O. Biçen, Y. Şişman, D. Hür, A. Denizli and A. Ersöz, Process Biochem., 2011, 46, 1688–1692 CrossRef CAS PubMed
.
- FEMA (Flavour and Extract Manufacturers Association), Food Technology, Part 2, 1965, vol. 19, pp. 151–197 Search PubMed
.
- FDA (Food and Drug Administration), Code of Federal Regulations, 21 CFR 172.515, Title 21-Food and Drugs, Chapter I - Food and Drug Administration, Department of Health and Human Services. Part 172-Food Additives Permitted for Direct Addition to Food for Human Consumption, Subpart F - Flavoring Agents and Related Substances, 515-Synthetic Flavoring Substances and Adjuvants, volume 3.
- JECFA (Joint Expert Committee on Food Additives), 2001, Safety evaluation of certain food additives. Who Food Additives Series: 48, Prepared by the fifty seventh meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), World Health Organization, Geneva 2001.
- IFRA (International Fragrance Association), Volume of Use Survey, September 2008 Search PubMed
.
- EPA (Environmental Protection Agency), United States Environmental Protection Agency, Washington, DC, USA, 2010.
- B. G. Davis, Annu. Rep. Prog. Chem., Sect. B: Org. Chem., 2003, 99, 49–62 RSC
.
- S. H. Jadhav and P. R. Gogate, Ind. Eng. Chem. Res., 2014, 53, 1377–1385 CrossRef CAS
.
- H. Ma, L. Huang, J. Junqiang, H. Ronghai, H. L. Lin and Z. Wenxue, Ultrason. Sonochem., 2011, 18, 419–424 CrossRef CAS PubMed
.
- H. Hirakawa, N. Kamiya, Y. Kawarabayashi and T. Nagamun, Biochim. Biophys. Acta, 2005, 1748, 94–99 CrossRef CAS PubMed
.
- D. Menezes-Blackburna, M. Jorquer, M. Gianfreda, R. Rao, E. Greiner and M. L. Garrido, Bioresour. Technol., 2011, 102, 9360–9367 CrossRef PubMed
.
- K. C. Badgujar and B. M. Bhanage, Fuel Process. Technol., 2015, 138, 139–146 CrossRef CAS PubMed
.
- A. M. Gumel, M. S. M. Annuar, T. Heidelberg and Y. Chisti, Bioresour. Technol., 2011, 102, 8727–8732 CrossRef CAS PubMed
.
- K. C. Badgujar and B. M. Bhanage, Process Biochem., 2014, 49, 1304–1313 CrossRef CAS PubMed
.
- K. C. Badgujar and B. M. Bhanage, Process Biochem. DOI:10.1016/j.procbio.2015.04.019
.
Footnote |
| † Electronic supplementary information (ESI) available: It involves images of 1H NMR and 13C NMR spectra. See DOI: 10.1039/c5ra10032a |
| This journal is © The Royal Society of Chemistry 2015 |