DOI:
10.1039/C5RA09974A
(Paper)
RSC Adv., 2015,
5, 63393-63400
Photocatalytic mineralization of anionic dyes using bismuth doped CdS–Ta2O5 composite†
Received
27th May 2015
, Accepted 17th July 2015
First published on 17th July 2015
Abstract
A stable and efficient bismuth doped CdS–Ta2O5 heterostructured system is synthesized for the photocatalytic degradation of anionic dyes like acid violet 7, indigo carmine and methyl orange. This composite is found to be effective not only for the degradation but also for the mineralization of these dyes under sunlight type irradiation. Bi doping improves the optical absorption property and enhances the visible light absorption. In this composite, CdS exists as the hexagonal wurtzite structure and Ta2O5 has an orthorhombic structure. Increased fluorescence lifetime is observed for the charge carriers in the composite sample compared to CdS due to the efficient charge separation occurring in this system. The enhanced photocatalytic activity of the composite system arises due to the improved optical absorption properties and increased life time of the photogenerated charge carriers. This heterostructured catalyst is found to be photostable and reusable. Photocatalytic degradation experiments with different quenchers suggest that hydroxyl radicals (OH˙), superoxide anion radicals (O2˙−) and photogenerated holes (h+) play significant roles in the mineralization process.
1. Introduction
Semiconductor photocatalysis is gaining much importance as it is one of the promising methods to deal with issues like environmental pollution and energy generation. Environmental pollution by organic pollutant discharge from textile and fine chemical industries is a serious issues due to various health hazards on terrestrial as well as aquatic biota. The efficient removal and degradation of these pollutants is a challenging task. Photocatalytic degradation of organic pollutants present in water is widely studied for treating polluted water. This process is attractive as solar radiation can be used for this purpose, which is a renewable energy source. Development of visible light active photocatalysts is of utmost importance for this application as nearly 45% of solar radiation consists of visible light. CdS is a visible light active catalyst having a bandgap of ∼2.4 eV and can be used for this reaction.1–3 For its effective use, certain disadvantages like photoinstability and fast recombination of photogenerated charge carriers have to be overcome.4,5 Dispersing CdS on another material can improve its stability. Besides, if the conduction band/valence band potentials of the material is suitable, photogenerated electrons/holes can be injected to it from CdS improving the charge separation and lifetime of the charge carriers.6–9
Heterostructured CdS composites are reported to be better systems for photocatalytic reactions than single phase CdS. Composites of CdS like, CdS–ZnO,10,11 CdS–TiO2,12,13 CdS–ZrO2,14,15 CdS–SnO2,16 CdS–CdO,17,18 CdS–zeolite19,20 and CdS–PANI (polyaniline)21 show improved photocatalytic activity compared to single phase CdS. In most of the cases, the enhanced activity is due to the increased surface area of the composite catalyst, improved separation of photogenerated charge carriers and increased stability of the composites. In a recent communication, a mesostructured CdS–Ta2O5 composite is reported to show enhanced photocatalytic activity for hydrogen generation from water.22 The reason for the enhancement is attributed to the efficient utilization of photogenerated charges.
In the present work, we report the photocatalytic activity of a heterostructured bismuth doped CdS dispersed on Ta2O5 for the degradation and mineralization of anionic dyes like acid violet 7 (AV 7), indigo carmine (IC) and methyl orange (MO). Two different strategies are employed to enhance the photocatalytic activity of CdS. Doping of CdS with Bi can improve the optical absorption property whereas dispersing the doped CdS on Ta2O5 can increase the lifetime of the photogenerated charge carriers and increase the surface area of the composite catalyst. This is the first report of the mineralization of anionic dyes using this system. A detailed characterization of the composite catalyst has been carried out and correlated the observed photocatalytic activity with the physico-chemical properties of this modified CdS system.
2. Experimental section
2.1. Materials
All chemicals were of analytical grade and were used as received without further purification. Tantalum(V) oxide (Alfa Aesar, 99.85%, metal basis, −325 mesh power), cadmium chloride hydrated AR (s.d. fine chem. Ltd 99.5%), sodium sulfide flakes purified (anhydrous), bismuth(III) nitrate pentahydrate (Aldrich, 99.999%, trace metal basis), thiourea GR (Merck, 99%), urea (Qualigens, 99%), ethylene glycol (Merck, 99%), HCl (Fisher Scientific). AV 7, IC and MO dyes were procured from Sigma-Aldrich. Millipore water was used in the preparation of all solutions.
2.2. Preparation of photocatalysts
Pure CdS was prepared by treating cadmium chloride with sodium sulfide in ethylene glycol medium.14 Briefly, cadmium chloride (4 g) dissolved in water was mixed with EG (total volume = 50 cm3, water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG = 1
EG = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) and refluxed at 100 °C for 15 minutes. At this stage, sodium sulfide (2 g) dissolved in water and EG (50 cm3, water
1 by volume) and refluxed at 100 °C for 15 minutes. At this stage, sodium sulfide (2 g) dissolved in water and EG (50 cm3, water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG = 1
EG = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) was added to the solution and refluxed at 120 °C for 4 h. The precipitate obtained was separated by centrifugation, washed and dried in an oven at 90 °C for 5 h followed by calcination in air at 350 °C for 2 h. Bismuth doped CdS (BiCdS) was synthesized by taking calculated amount of bismuth nitrate (atomic weight) dissolved in 1 M HNO3 (5 ml) and adopting the same procedure as for CdS. For the synthesis of CdS–Ta2O5 composite (XXCT, where XX stand for the percentage of CdS in the composite), analytical grade Ta2O5 (Alpha Acer) was used. Different amounts of CdS (% by weight) were loaded on Ta2O5 by an impregnation method. Aqueous cadmium chloride solution was stirred with Ta2O5 powder and evaporated to dryness (80–90 °C). The cadmium chloride adsorbed on Ta2O5 was heated with an aqueous solution of thiourea (Cd
1 by volume) was added to the solution and refluxed at 120 °C for 4 h. The precipitate obtained was separated by centrifugation, washed and dried in an oven at 90 °C for 5 h followed by calcination in air at 350 °C for 2 h. Bismuth doped CdS (BiCdS) was synthesized by taking calculated amount of bismuth nitrate (atomic weight) dissolved in 1 M HNO3 (5 ml) and adopting the same procedure as for CdS. For the synthesis of CdS–Ta2O5 composite (XXCT, where XX stand for the percentage of CdS in the composite), analytical grade Ta2O5 (Alpha Acer) was used. Different amounts of CdS (% by weight) were loaded on Ta2O5 by an impregnation method. Aqueous cadmium chloride solution was stirred with Ta2O5 powder and evaporated to dryness (80–90 °C). The cadmium chloride adsorbed on Ta2O5 was heated with an aqueous solution of thiourea (Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thiourea = 1
thiourea = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, 80–90 °C). The resultant mixture was then evaporated to dryness followed by heating the powder at 350 °C for 2 h. The bismuth doping for Cd (0.5%, 1% and 2% by atomic weight) in 50CT composite (named as XBi50CT, where X stands for Bi concentration) was prepared by mixing calculated amount of bismuth nitrate solution in 1 M HNO3 (5 ml) along with cadmium chloride solution and repeating the same procedure mentioned above for the preparation of CT composites.
4 ratio, 80–90 °C). The resultant mixture was then evaporated to dryness followed by heating the powder at 350 °C for 2 h. The bismuth doping for Cd (0.5%, 1% and 2% by atomic weight) in 50CT composite (named as XBi50CT, where X stands for Bi concentration) was prepared by mixing calculated amount of bismuth nitrate solution in 1 M HNO3 (5 ml) along with cadmium chloride solution and repeating the same procedure mentioned above for the preparation of CT composites.
2.3. Photocatalytic experiments
The photocatalytic activity of the composite catalysts was studied for the degradation of different anionic dyes like acid violet 7 (AV 7), methyl orange (MO) and indigo carmine (IC). For each experiment, 125 ml of dye (50 ppm) solution was taken in a beaker containing 0.35 g of catalyst suspended in it. The experiments were carried out in a homemade photoirradiator using day light fluorescent lamp (Oreva, 12 lamps, 100 W each) as source of radiation. The radiation from the lamps contains mainly visible light along with <3% UV light.23,24 Irradiator consists of 12 fluorescent lamps fitted vertically and equidistantly at the base of a rectangular galvanized iron chamber with aluminium foil on the walls of box as a reflecting media. Air circulation was carried out to nullify the heating effect. The average light flux measured by lutron lux meter (model LX-101) was found out to be 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–65
000–65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lux. Before irradiation, the catalyst suspension in the dye solution was kept in the dark for 30 minutes under constant stirring to establish the adsorption–desorption equilibrium at ambient conditions. During irradiation, magnetic stirring was performed for the homogenization of suspension. For the photocatalytic activity measurement, a small volume of clear supernatant liquid was taken at regular time intervals, centrifuged it and analyzed with UV-visible spectrophotometer (Shimadzu, UV-1800). The concentration of AV 7 was monitored by taking its absorbance at λmax = 523 nm.
000 lux. Before irradiation, the catalyst suspension in the dye solution was kept in the dark for 30 minutes under constant stirring to establish the adsorption–desorption equilibrium at ambient conditions. During irradiation, magnetic stirring was performed for the homogenization of suspension. For the photocatalytic activity measurement, a small volume of clear supernatant liquid was taken at regular time intervals, centrifuged it and analyzed with UV-visible spectrophotometer (Shimadzu, UV-1800). The concentration of AV 7 was monitored by taking its absorbance at λmax = 523 nm.
For checking photostability, the same catalyst was reused for repeated cycles of experiment after one cycle of photocatalytic reaction. After each run, the used catalysts from the suspension was removed by centrifugation and washed thrice with water and acetone followed by drying in vacuum oven at 90 °C for 2 h. The total organic carbon of the dye solution at different time intervals of the photocatalytic reaction was analyzed with the TOC analyzer (Shimadzu, TOC-L). TOC value was calculated by taking the difference between the total carbon and the inorganic carbon.
2.4. Characterization
X-ray powder diffraction (XRD) patterns were obtained using a X'Pert PRO PANalytical X-ray diffractometer (Philips, Holland) with Cu Kα radiation (α = 1.5418 Å) at 40 kV. The surface area was determined by Brunauer–Emmet–Teller (BET) method using Micromeritics ASAP 2020 V3.04 H surface area analyzer by nitrogen adsorption at 77 K. The TEM images were taken using a FEI Tecnai T-20 electron microscope operating at 300 kV. Energy dispersive X-ray spectroscopy (EDXS) analysis was carried out using FEI Quanta 200 ESEM. UV-visible diffuse reflectance spectra (UV-vis DRS) of all samples were recorded using a Jasco (model V-670) spectrophotometer equipped with an integrating sphere accessory. Barium sulfate was used as reference for the reflectance spectra. Photoluminescence (PL) spectra were recorded using FLSP 920 (Edinburgh Instruments) equipped with a 450 W Xe arc lamp as the excitation source and a red sensitive Peltier element cooled Hamamatsu R2658 PMT as the detector. Excited state lifetimes of the charge carriers were measured with the same instrument using a nanosecond hydrogen flash lamp as the excitation source and employing Time Correlated Single Photon Counting (TCSPC) technique.
3. Results and discussion
3.1. Characterization
Fig. 1 shows the powder X-ray diffraction patterns of CdS, 1BiCdS, Ta2O5, 50CT, and 1Bi50CT. It can be seen from the figure that pure CdS and Ta2O5 are monophasic with no other impurity phases. Pure CdS shows the wurtzite type (hexagonal) structure (JCPDS file no. 01-075-1545) and Ta2O5 has orthorhombic structure (JCPDS file no. 01-071-0639). The pattern of Bi doped CdS is similar to that of pure CdS. XRD patterns of 50CT and 1Bi50CT show peaks corresponding to both CdS and Ta2O5 confirming that they exist as composites. Peaks corresponding to oxide or sulfide of bismuth are not seen in 1BiCdS or 1Bi50CT suggesting that Bi might have substituted for Cd in the lattice sites. But, as the ionic size of Bi3+ (96 pm) is higher than that of Cd2+ (78 pm), it is likely that surface doping takes place in this case. XRD patterns of other compositions, 29CT and 70CT along with CdS and Ta2O5 are given in Fig. S1 of ESI.† All composites show peaks corresponding to CdS and Ta2O5 indicating that they exist in biphasic form. Powder XRD patterns of Bi doped CdS–Ta2O5 composites containing different concentrations of Bi are shown in Fig. S2 of ESI.† All patterns are identical and exhibit peaks corresponding to CdS and Ta2O5 phases.
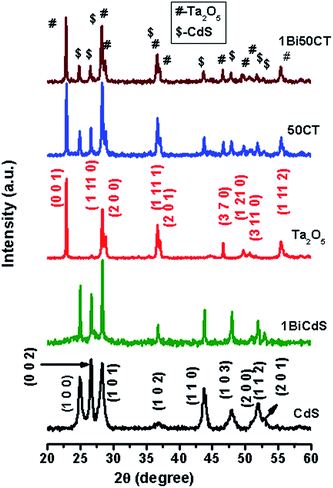 |
| | Fig. 1 XRD patterns of CdS, 1BiCdS, Ta2O5, 50CT and 1Bi50CT. | |
Fig. S3 (ESI†) shows the SEM images of CdS, Ta2O5 and 1Bi50CT. SEM image of pure CdS shows a large particle size distribution of ∼1 to 7 microns. Ta2O5 shows more or less uniform particle size in the range of 1–3 microns. The image of composite exhibits an irregular morphology with non-uniform particle size. Fig. 2 shows the TEM image, HRTEM and SAED pattern of 1Bi50CT composite. TEM image shows particles with size in the range of ∼50–250 nm. SAED pattern exhibits the presence of both Ta2O5 and CdS indicating the dispersed state of the composite. HRTEM images show lattice fringes with d spacing corresponding to both CdS and Ta2O5, which further confirms the dispersed state and biphasic nature of the composite. EDAX spectrum of the composite sample (Fig. S4, ESI†) suggests that the concentration of Bi in the composite is ∼1.2% (by weight). To see the distribution of elements presents in 1Bi50CT heterostructures, energy dispersive X-ray spectroscopy (EDS) with elemental mapping was performed and the images are shown in Fig. S5 of ESI.† The mapping results of selected area of SEM image suggest the coexistence of Bi, Cd, S, O and Ta elements in the heterostructures. The images show no clustering of a particular component indicating that all elements are randomly distributed and in a dispersed state.
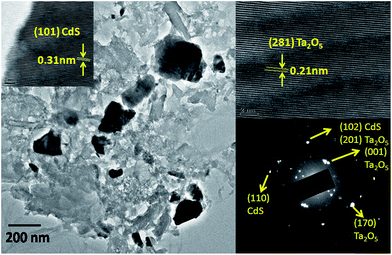 |
| | Fig. 2 TEM image, HRTEM image and SAED pattern of 1Bi50CT. | |
3.2. Optical properties
Fig. 3A shows the UV-visible DRS of CdS, Ta2O5, 1BiCdS, 50CT and 1Bi50CT. The spectra of other compositions are given in Fig. S6 of ESI.† A significant red shift of the absorption edge is observed for the composite when compared to pure Ta2O5. The shift of the composite can be due to the interaction of CdS and Ta2O5 at the interface forming Cd–O–Ta type bonding.25 The spectra of 1BiCdS and 1Bi50CT show a slight red shift of the absorption edge and improved visible light absorption compared to 50CT. This can be due to the introduction of additional energy levels within the bandgap of CdS as a result of Bi doping, which decreases the energy gap of CdS. Band gap energy of CdS and the composites has been calculated from the transformed Kubleka–Munk plots of (F(R)hν)2 vs. photon energy Fig. 3B and the values are given Table 1. A noticeable change in the bandgap energy is observed for 1BiCdS and 1Bi50CT.
 |
| | Fig. 3 (A) UV-visible DRS spectra of CdS, Ta2O5, 1BiCdS, 50CT and 1Bi50CT. (B) Kubelka–Munk plot for band gap calculation of CdS, 1BiCdS, 50CT and 1Bi50CT. | |
Table 1 Kinetic data for the photocatalytic degradation of AV 7 (50 ppm) and band gap values of prepared catalysts
| S. No. |
Catalysts |
Rate K (min−1) (30 min) |
Degradation efficiency (%) (60 min) |
Band gap (eV) |
| 1 |
CdS |
0.016 |
64.79 |
2.37 |
| 2 |
Ta2O5 |
— |
0.99 |
3.83 |
| 3 |
1BiCdS |
0.019 |
73.34 |
2.32 |
| 4 |
29CT |
0.007 |
37.18 |
2.33 |
| 5 |
50CT |
0.067 |
99.59 |
2.35 |
| 6 |
70CT |
0.031 |
96.35 |
2.36 |
| 7 |
1Bi50CT |
0.105 |
99.88 |
2.30 |
Photoluminescence (PL) spectra of some representative samples were recorded to probe the nature of emission occurring from the composite samples and defect levels present in them. PL spectrum of CdS (Fig. 4A) shows a broad peak starting from 505 nm, which extends beyond 600 nm. Comparing the peak position with the bandgap value of 2.37 eV, it can be assigned to a combined peak due to band edge emission as well as emission from defect levels.26 The emission peak of 1BiCdS is similar to that of CdS except that it is slightly red shifted. This is due to the alteration of bandgap energy as a result of doping. PL spectrum of Ta2O5 shows a peak centered around 410 nm, which can be assigned to emission from defect levels as it is a wide bandgap material. The bandgap value calculated from the absorption spectrum of this sample is 3.8 eV. The spectra of the composites show features of both CdS and Ta2O5. The defect emission peak due to Ta2O5 is broader for the composites indicating that the nature of defect levels and their energetics have changed in the composites.
 |
| | Fig. 4 (A) PL spectra of CdS, 1BiCdS, Ta2O5, 50CT and 1Bi50CT. (B) Fluorescence emission decay curves of CdS, 1BiCdS, 50CT and 1Bi50CT. | |
The fluorescence lifetime, which is a measure of the lifetime of the photogenerated charge carriers, has been calculated from the time resolved fluorescence spectra. Lifetime of CdS and the CdS containing composites was studied for the emission occurring at 530 nm as it is occurring from CdS and the excitation wavelength employed was 282 nm. It can be seen from Fig. 4B that the fluorescence decay is slower for the composites compared to CdS. The lifetime values obtained by fitting the curves are 0.80, 0.85, 3.8 and 4.2 ns for CdS, 1BiCdS, 50CT and 1Bi50CT respectively. These values suggest that recombination of photogenerated charge carriers in the composite is slower, which can enhance the photocatalytic activity.
3.3. XPS analysis of the composite
X-ray photoelectron spectra of CdS and the composites were recorded to see the oxidation state of different elements and the presence of different surface species. Cd 3d spectra of CdS, 50CT and 1Bi50CT are shown in Fig. 5. Spectrum of pure CdS shows peaks at 404.8 and 411.7 eV corresponding to the 3d5/2 and 3d3/2 spin–orbit components of Cd 3d respectively. These peak positions correspond to the 2+ oxidation state of Cd in CdS.27,28 The corresponding peak positions of Cd in the spectra of composites are almost the same as that of CdS. Peak positions for S 2p binding states for all samples are shown in Fig. 5. Binding energy values of S 2p peak of CdS is 162.5 eV and correspond to the S2− state of S in CdS.29–31 There is no significant change in the peak positions of S 2p in the spectra of the composites suggesting that sulfur exists as S2− in the composites. Peak due to Bi could not be seen in the spectrum of 1Bi50CT as the concentration of Bi is just 1% in the composite.
 |
| | Fig. 5 Cd 3d and S 2p XPS spectra of CdS, 50CT and 1Bi50CT. | |
3.4. Photocatalytic degradation of AV 7 dye
Fig. 6A elucidates the photocatalytic behaviour of CdS–Ta2O5 composites having different concentrations of CdS for the degradation of AV 7 (50 ppm). It can be seen from the figure that the photocatalytic activity increases as the CdS concentration increases. Among the CdS loaded samples, 50CT shows the highest activity and degradation efficiency. The lower activity of the composite containing lower concentrations of CdS can be due to the dilution effect of photoactive CdS as Ta2O5 cannot be excited by the light source used here. At higher concentrations, aggregation of CdS can occur, which decreases the photocatalytic activity. Doping 50CT with Bi enhances the photocatalytic activity further. Photocatalytic activity of Bi50CT composite having different concentrations of Bi such as 0.5, 1 and 2% is shown in Fig. 6B. It can be seen from the figure that 1Bi50CT shows the highest degradation efficiency when compared with the other composites and CdS. Relative efficiencies of different catalysts including CdS, 50CT composite and 1Bi50CT along with the results of blank and dark experiments are shown in Fig. 7A. Photocatalytic activity for the degradation AV 7 decreased in the order: 1Bi50CT > 50CT > 1BiCdS > CdS > Ta2O5. The degradation efficiency and the rate constant for the reaction calculated for the initial reaction period of 60 and 30 minutes are presented in Table 1. It is clear from Table 1 that 1Bi50CT shows the highest degradation efficiency and degradation rate compared to CdS and other composites. Hence, further experiments are conducted using 1Bi50CT catalyst. The degradation efficiency and the rate constant for the degradation reaction of AV 7 for different Bi doped CdS–Ta2O5 samples are given Table S7 of ESI.†
 |
| | Fig. 6 (A) Effect of CdS loading on Ta2O5 (weight%) for photocatalytic degradation of AV 7. (B) Effect of Bi doping (atomic%) in 50CT for photocatalytic degradation of AV 7. | |
 |
| | Fig. 7 (A) Photocatalytic performance of prepared catalysts for 50 ppm AV 7. (B) Total organic carbon (TOC%) during photocatalytic degradation reaction of AV 7 by 1Bi50CT. | |
In order to confirm whether just decolourization occurs or the mineralization of the dye takes place during the photocatalytic reaction using 1Bi50CT, total organic carbon (TOC) of the solution was evaluated at regular intervals of illumination. The TOC values are plotted against irradiation time and are shown in Fig. 7B. It can be seen that the total organic carbon has decreased almost to zero after irradiation for 30 minutes. Thus, it is clear from the figure that it is not just decolourization but mineralization of the dye occurs during photocatalytic reaction.
The improved photocatalytic activity of 1Bi50CT samples can be attributed to the improved visible light absorption and increased lifetime of the photogenerated charge carriers in the composite system. As the conduction band (CB) potential of Ta2O5 is lower than that of CdS,32–34 photogenerated electrons from the CB of CdS can be injected to the CB of Ta2O5 at the interface of the two compounds in the composite material. Thus, the favourable heterojunctions formed in the composite enhances the charge separation and improves the photocatalytic activity. This view is supported by the fluorescence lifetime studies of these composites. Bi doping enhances the photocatalytic activity of the composite further due to the improved visible light absorption. A schematic of the possible electron transfer process occurring in this system is shown in Fig. 8. The BET surface areas of CdS, Ta2O5 and 1Bi50CT are 0.4, 2.7 and 1.1 m2 g−1 respectively. The improved surface area of the 1Bi50CT compared to pure CdS can also contribute to the enhanced photocatalytic activity of the composite sample.
 |
| | Fig. 8 Schematic illustration of the photogenerated electron transfer process in 1Bi50CT. | |
3.4.1. Mechanistic study. In order to understand the active species responsible for the photocatalytic degradation of AV 7 over 1Bi50CT, the degradation experiment was performed in the presence of different quenchers. Quenchers are certain chemicals which hinder the action of certain specific species for the degradation reaction by trapping them during the course of photocatalytic experiment. The different quenchers used are ammonium oxalates to trap photogenerated holes (h+), p-benzoquinone for superoxide radical anions (O2˙−) and iso-propanol for hydroxyl radicals (OH˙).35–38 The variation of the concentration of dye as a function of irradiation time in the presence of quenchers is shown in Fig. 9A. It can be seen from the figure that in the presence of quenchers, the photocatalytic activity has decreased significantly. The degradation efficiency in the presence of different quenchers is shown in Fig. 9B. These results indicate that all three species play active roles in the photocatalytic degradation of AV 7. In the light of the above results, the possible reactions for the degradation of AV 7 are given below.
| CdS(Bi) − Ta2O5 + hν → CdS(Bi) − Ta2O5(e−CB⋯h+VB) |
| CdS(Bi)(e−CB) + Ta2O5 → CdS(Bi) + Ta2O5(e−CB) |
| AV 7 + CdS(Bi)(h+VB) → degradation products |
where e−CB and h+VB stand for electron in the conduction band and hole in the valence band, respectively.
 |
| | Fig. 9 (A) Effect of quenchers on the photocatalytic degradation of AV 7 by 1Bi50CT. (B) Effect of quenchers on degradation efficiency (%, 45 min) of AV 7 by 1Bi50CT. | |
When the composite is irradiated with photon energy exceeding the bandgap of the semiconductor, the photogenerated charge carriers, electron and holes, are generated. Electron from conduction band of CdS can get transferred to the conduction band of Ta2O5 leaving behind positively charged hole. The holes and electron produce hydroxyl radicals (OH˙) and active oxygen radical species, like superoxide radical anions (O2˙−) respectively and degrade the AV 7 dye. The holes also interact with the AV 7 leads to its complete degradation which was further supported by TOC results.
3.4.2. Stability of the catalyst. The stability of 1Bi50CT photocatalyst was checked by running the degradation experiment for repeated cycles using the same catalyst. The results are shown in Fig. S8 (ESI).† From the figure, it is clear that there is only a slight decrease in the photocatalytic activity with repeated cycles indicating that the catalyst is stable and can be reused. The slight decrease in the photocatalytic activity can be due to the loss of the catalysts during the washing and drying operation. It can be seen from Fig. S9† that the dark adsorption of the dye on the catalyst decreases with repeated cycles suggesting that a small part of the dye or degraded products remain adsorbed on the catalyst. However, it may be noticed that at the end of 60 min, dye was not detected in the solution indicating that the dye present in the solution gets degraded completely. Phase stability of the catalysts was checked by recording the XRD pattern after prolonged use. The XRD pattern of the used catalyst is identical to the fresh catalyst (Fig. S10, ESI†) suggesting that the catalyst is quite stable and no phase change occurs during photocatalytic reaction.
3.5. Photocatalytic degradation of other anionic dyes using 1Bi50CT
The photocatalytic activity of 1Bi50CT for the degradation and mineralization of other anionic dyes like indigo carmine (IC, 50 ppm) and methyl orange (MO, 20 ppm) was also studied. The results are shown in Fig. 10. It can be seen that the composite shows enhanced photocatalytic activity compared to CdS and Ta2O5. Besides, the Bi doped composite is quite efficient for both the degradation and mineralization of these dyes, as seen from the TOC analysis results. The degradation rates and efficiencies are given in Table S11 of ESI.† Higher values of rate constant are observed for both these dyes using this catalyst and this system works quite efficiently for all the anionic dyes studied.
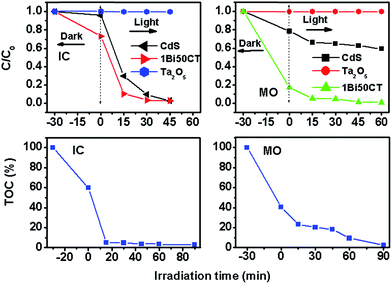 |
| | Fig. 10 Photocatalytic performance of CdS, Ta2O5 and 1Bi50CT for other anionic dyes as IC (50 ppm) and MO (20 ppm) and total organic carbon (TOC%) during photocatalytic degradation reaction of IC (50 ppm) and MO (20 ppm) by 1Bi50CT. | |
4. Conclusions
Bi doped CdS dispersed on Ta2O5 exhibits enhanced photocatalytic activity for the degradation of anionic dyes such as acid violet 7 (AV 7), methyl orange (MO) and indigo carmine (IC). Not just degradation, but mineralization of these dyes occurs during the photocatalytic reaction. The active species responsible for the degradation are found to be photogenerated holes (h+), O2˙− and OH˙ radicals. Bi doping in CdS results in the decrease of bandgap resulting in increased visible light absorption by Bi doped CdS–Ta2O5 composite. Increased fluorescence lifetime for the photogenerated charge carriers is observed for the composite sample compared to pure CdS due to the transfer of photogenerated electrons from the conduction band of CdS to the conduction band of Ta2O5. The enhanced photocatalytic activity of the composite is attributed to the improved visible light absorption and increased lifetime of the photogenerated charge carriers in the composite. The catalyst is quite stable and can be used for a prolonged period of time.
Acknowledgements
SSU is thankful to DAE-BRNS, Mumbai for financial support through major research project no. 2010/37c/28/BRNS 1426. Authors are thankful to Dr (Mrs) S. R. Bharadwaj, Head, Fuel Cell Materials and catalysis section, for the constant encouragement provided during the course of this work. One of the authors Sachin G. Ghugal is thankful to Visvesvaraya National Institute of Technology (VNIT), Nagpur for awarding the Ph. D. fellowship.
References
- Y. Shi, H. Li, L. Wang, W. Shen and H. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 4800–4806 CAS.
- R. Sahoo, R. Anindita, C. Ray, C. Mondal, Y. Negishi, S. M. Yusuf, A. Pal and T. Pal, J. Phys. Chem. C, 2014, 118, 11485–11494 CAS.
- C. J. Lin, Y. H. Yu and Y. H. Liou, Appl. Catal., B, 2009, 93, 119–125 CrossRef CAS.
- F. Yang, N. N. Yan, S. Huang, Q. Sun, L. Z. Zhang and Y. Yu, J. Phys. Chem. C, 2012, 116, 9078–9084 CAS.
- H. Zhang and Y. Zhu, J. Phys. Chem. C, 2010, 114, 5822–5826 CAS.
- S. Khanchandani, S. Kundu, A. Patra and A. K. Ganguli, J. Phys. Chem. C, 2013, 117, 5558–5567 CAS.
- S. Balachandran and M. Swaminathan, J. Phys. Chem. C, 2012, 116, 26306–26312 CAS.
- S. Khanchandani, S. Kundu, A. Patra and A. K. Ganguli, J. Phys. Chem. C, 2012, 116, 23653–23662 CAS.
- P. Kundu, P. A. Deshpande, G. Madras and N. J. Ravishankar, Mater. Chem. Phys., 2011, 21, 4209–4216 CAS.
- R. Sasikala, A. P. Gaikwad, V. Sudarsana, N. Gupta and S. R. Bharadwaj, Appl. Catal., A, 2013, 464–465, 149–155 CrossRef CAS.
- X. Yang, Q. Yang, Z. Hu, S. Guo, Y. Li, J. Sun, N. Xu and J. Wu, Sol. Energy Mater. Sol. Cells, 2015, 137, 169–174 CrossRef CAS.
- Z. Wei, Y. Li, S. Luo, C. Liu, D. Meng, M. Ding and G. Zeng, Sep. Purif. Technol., 2014, 122, 60–66 CrossRef CAS.
- L. Li, L. Wang, T. Hu, W. Zhang, X. Zhang and X. Chen, J. Solid State Chem., 2014, 218, 81–89 CrossRef CAS.
- R. Sasikala, A. R. Shirole, V. Sudarsan, K. G. Girija, R. Rao, C. Sudakar and S. R. Bharadwaj, J. Mater. Chem., 2011, 21, 16566–16573 RSC.
- L. Li, L. Wang, W. Zhang, X. Zhang, X. Chen and X. Dong, J. Nanopart. Res., 2014, 16, 1–14 CAS.
- S. G. Ghugal, S. S. Umare and R. Sasikala, Appl. Catal., A, 2015, 496, 25–31 CrossRef CAS.
- S. V. Kahane, R. Sasikala, B. Vishwanadh, V. Sudarsan and S. Mahamuni, Int. J. Hydrogen Energy, 2013, 38, 15012–15018 CrossRef CAS.
- W. Yang, Y. Liu, Y. Hu, M. Zhou and H. Qian, J. Mater. Chem., 2012, 22, 13895–13898 RSC.
- R. Sasikala, A. P. Gaikwad, V. Sudarsan, R. Rao, Jagannath, B. Viswanadh and S. R. Bharadwaj, Phys. Chem. Chem. Phys., 2015, 17, 6896–6904 CAS.
- X. Zhou, H. Chen, Y. Sun, K. Zhang, X. Fan, Y. Zhu, Y. Chen, G. Tao and J. Shi, Appl. Catal., B, 2014, 152–153, 271–279 CrossRef CAS.
- K. He, M. Li and L. Guo, Int. J. Hydrogen Energy, 2012, 37, 755–759 CrossRef CAS.
- L. Xu, W. Shi and J. Guan, Catal. Commun., 2012, 25, 54–58 CrossRef CAS.
- V. N. Kuznetsov and N. Serpone, J. Phys. Chem. B, 2006, 110, 25203–25209 CrossRef CAS PubMed.
- C. Belver, R. Bellod, S. J. Stewart and F. G. Requejo, Appl. Catal., B, 2006, 65, 309–314 CrossRef CAS.
- H. Kisch and H. Weiß, Adv. Funct. Mater., 2002, 12, 483–488 CrossRef CAS.
- B. Yang, J. E. Schneeloch, Z. Pan, M. Furis and M. Achermann, Phys. Rev. B., 2010, 81, 073401 CrossRef.
- H. Khallaf, C. Chen, L. Chang, O. Lupan, A. Dutta, H. Heinrich, A. Shenouda and L. Chow, Appl. Surf. Sci., 2011, 257, 9237–9242 CrossRef CAS.
- L. Wu, J. C. Yu and X. J. Fu, J. Mol. Catal. A: Chem., 2006, 244, 25–32 CrossRef CAS.
- N. Zhang, S. Liu, X. Fu and Y. J. Xu, J. Mater. Chem., 2012, 22, 5042–5052 RSC.
- S. Rengaraj, S. Venkataraj, S. H. Jee, Y. Kim, C. W. Tai, E. Repo, A. Koistinen, A. Ferancova and M. Sillanpaa, Langmuir, 2010, 27, 352–358 CrossRef PubMed.
- Z. Chen and Y. J. Xu, ACS Appl. Mater. Interfaces, 2013, 5, 13353–13363 CAS.
- L. Xu, W. Shi and c Guan, Catal. Commun., 2012, 25, 54–58 CrossRef CAS.
- Y. Xu and M. A. A. Schoonen, Am. Mineral., 2000, 85, 543 CrossRef CAS.
- W. J. Chun, A. Ishikawa, H. Fujisawa, T. Takata, J. N. Kondo, M. Hara, M. Kawai, Y. Matsumoto and K. Domen, J. Phys. Chem. B, 2003, 107, 1798 CrossRef CAS.
- S. Kumar, S. Khanchandani, M. Thirumal and A. K. Ganguli, ACS Appl. Mater. Interfaces, 2014, 6, 13221–13233 CAS.
- S. G. Ghugal, S. S. Umare and R. Sasikala, Mater. Res. Bull., 2015, 61, 298–305 CrossRef CAS.
- K. H. Reddy, S. Martha and K. M. Parida, Inorg. Chem., 2013, 52, 6390–6401 CrossRef CAS PubMed.
- S. Khanchandani, P. K. Srivastava, S. Kumar, S. Ghosh and A. K. Ganguli, Inorg. Chem., 2014, 53, 8902–8912 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09974a |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG = 1
EG = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) and refluxed at 100 °C for 15 minutes. At this stage, sodium sulfide (2 g) dissolved in water and EG (50 cm3, water
1 by volume) and refluxed at 100 °C for 15 minutes. At this stage, sodium sulfide (2 g) dissolved in water and EG (50 cm3, water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG = 1
EG = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) was added to the solution and refluxed at 120 °C for 4 h. The precipitate obtained was separated by centrifugation, washed and dried in an oven at 90 °C for 5 h followed by calcination in air at 350 °C for 2 h. Bismuth doped CdS (BiCdS) was synthesized by taking calculated amount of bismuth nitrate (atomic weight) dissolved in 1 M HNO3 (5 ml) and adopting the same procedure as for CdS. For the synthesis of CdS–Ta2O5 composite (XXCT, where XX stand for the percentage of CdS in the composite), analytical grade Ta2O5 (Alpha Acer) was used. Different amounts of CdS (% by weight) were loaded on Ta2O5 by an impregnation method. Aqueous cadmium chloride solution was stirred with Ta2O5 powder and evaporated to dryness (80–90 °C). The cadmium chloride adsorbed on Ta2O5 was heated with an aqueous solution of thiourea (Cd
1 by volume) was added to the solution and refluxed at 120 °C for 4 h. The precipitate obtained was separated by centrifugation, washed and dried in an oven at 90 °C for 5 h followed by calcination in air at 350 °C for 2 h. Bismuth doped CdS (BiCdS) was synthesized by taking calculated amount of bismuth nitrate (atomic weight) dissolved in 1 M HNO3 (5 ml) and adopting the same procedure as for CdS. For the synthesis of CdS–Ta2O5 composite (XXCT, where XX stand for the percentage of CdS in the composite), analytical grade Ta2O5 (Alpha Acer) was used. Different amounts of CdS (% by weight) were loaded on Ta2O5 by an impregnation method. Aqueous cadmium chloride solution was stirred with Ta2O5 powder and evaporated to dryness (80–90 °C). The cadmium chloride adsorbed on Ta2O5 was heated with an aqueous solution of thiourea (Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thiourea = 1
thiourea = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, 80–90 °C). The resultant mixture was then evaporated to dryness followed by heating the powder at 350 °C for 2 h. The bismuth doping for Cd (0.5%, 1% and 2% by atomic weight) in 50CT composite (named as XBi50CT, where X stands for Bi concentration) was prepared by mixing calculated amount of bismuth nitrate solution in 1 M HNO3 (5 ml) along with cadmium chloride solution and repeating the same procedure mentioned above for the preparation of CT composites.
4 ratio, 80–90 °C). The resultant mixture was then evaporated to dryness followed by heating the powder at 350 °C for 2 h. The bismuth doping for Cd (0.5%, 1% and 2% by atomic weight) in 50CT composite (named as XBi50CT, where X stands for Bi concentration) was prepared by mixing calculated amount of bismuth nitrate solution in 1 M HNO3 (5 ml) along with cadmium chloride solution and repeating the same procedure mentioned above for the preparation of CT composites.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–65
000–65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lux. Before irradiation, the catalyst suspension in the dye solution was kept in the dark for 30 minutes under constant stirring to establish the adsorption–desorption equilibrium at ambient conditions. During irradiation, magnetic stirring was performed for the homogenization of suspension. For the photocatalytic activity measurement, a small volume of clear supernatant liquid was taken at regular time intervals, centrifuged it and analyzed with UV-visible spectrophotometer (Shimadzu, UV-1800). The concentration of AV 7 was monitored by taking its absorbance at λmax = 523 nm.
000 lux. Before irradiation, the catalyst suspension in the dye solution was kept in the dark for 30 minutes under constant stirring to establish the adsorption–desorption equilibrium at ambient conditions. During irradiation, magnetic stirring was performed for the homogenization of suspension. For the photocatalytic activity measurement, a small volume of clear supernatant liquid was taken at regular time intervals, centrifuged it and analyzed with UV-visible spectrophotometer (Shimadzu, UV-1800). The concentration of AV 7 was monitored by taking its absorbance at λmax = 523 nm.










