Flexible, transparent and robust SERS tapes through a two-step block copolymer self-assembly process†
Vignesh Suresh and
Fung Ling Yap*
Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology, and Research (A*STAR), 3, Research Link, Singapore 117602. E-mail: yapfl@imre.a-star.edu.sg
First published on 25th June 2015
Abstract
Surface enhanced Raman spectroscopy (SERS) is an analytical technique that offers the capability of remote sensing, single molecule detection, and detection of trace contaminants (in parts per million) with high sensitivity and accuracy. Here, we demonstrate a simple and economical method for fabricating large area SERS-active tapes that are flexible, transparent and robust using a two-step process. The first is the fabrication of the gold nanoclusters on a flat chip using block copolymer self-assembly followed by directed electrostatic self-organization of gold nanoparticles (AuNPs). The second step involves the transfer of the resulting metal nanoclusters onto a thermal tape by a simple ‘stick and peel’ technique. Such substrates facilitate the detection and quantification of contaminants on irregular surfaces such as fruit skin, fabrics and other non-planar surfaces without the need to extract the analyte. Furthermore, the SERS measurements are highly quantitative, reproducible and the two-step fabrication process is unprecedented and has potential towards realizing large scale manufacturing of low-cost, flexible SERS-tape for on-field applications.
1. Introduction
Surface-enhanced Raman spectroscopy (SERS) has been an attractive analytical technique for applications that include remote sensing, single molecule detection, label-free detection, bio-sensing, forensics, pesticides and trace chemical detection.1–6 It offers an excellent platform to build low cost, versatile and large area SERS-active substrates due to its potential for high sensitivity and accuracy. Raman signals being extremely sensitive to the shape, size and arrangement of the metal structures on the substrate, the fabrication technique involved5–7 demands the need for precise control over feature dimensions and geometry. Furthermore, reproducibility in SERS signal enhancement is crucial to sensing and largely depends on the uniformity in substrate features that are achieved through various nanofabrication processes such as electron beam lithography, UV photolithography and Anodic Aluminium Oxide (AAO) arrays.8–13 Although, these techniques provide highly uniform pattern arrangements that in turn offer high and uniform signal enhancements, they compromise not only on the cost, simplicity in approach, and throughput but also in the adaptability of the techniques to fabricate flexible substrates for direct sensing of analytes on an irregular surface.Flexible SERS substrates are highly versatile in their utility and can be put in use onto irregular surfaces such as fabrics, fruit skin and in forensics.8,14–16 On the other hand, the flat, rigid chips fail to adapt to such conditions, since they demand the extraction of the analyte (using solvent) from non-planar surfaces prior to analysis which is not effective especially in detecting chemicals in trace quantities where the extraction is bound to result in material loss. To overcome these limitations, there are some reports on the fabrication of flexible SERS substrates made of cellulose paper, nanofiber mat and polymer support.16–20 The direct deposition of metal NPs on flexible substrates using various coating methods result in random distribution and varied density of NPs on the surface resulting in variation in SERS signal and thereby making the quantification of analyte difficult.21 Moreover, many of these supporting substrates either are not transparent22,23 or the transparency suffers after the addition of the SERS-active layer. The stability of these flexible materials towards harsh solvent and high energy laser also demands further investigation. Fiber-optic based SERS is an alternative, however, they involve high cost and is more suited for detection of chemicals in solution.2,3,24,25
To meet the needs for on-field detection, we demonstrate the fabrication of flexible SERS-active tapes in this report. They are obtained by pattern transfer of the SERS-active metal nano cluster arrays fabricated through a block copolymer (BCP) self-assembly process from flat chip on to a flexible tape through a ‘stick and peel’ technique. In this way, large area uniform metal nano cluster arrays on the tape that mirror the pattern-arrangement defined on the planar substrate is obtained. The ‘stick and peel’ method provides a route to precisely control AuNPs arrangement on flexible substrates, this cannot be easily achieve with any other direct fabrication methods on tape.
Self-assembly of block copolymer is often regarded as the technique to produce macroscopic, functional arrays of polymers with ultra-high density in the scale of tens of terabit per square inch.26,27 The BCP self-assembly not only allows for the ordered arrangement of the polymer features but also offers a handle to tune the geometry of the features, viz., the size, pitch and feature-to-feature spacing.26,28 Furthermore, the inherently functionalized features of the blocks provide the leverage to attach (either electrostatically or in situ) different material components and thereby extends the range of possible applications starting from memory devices to sensing through SERS.29–32 This simplicity offered by BCP self-assembly is exploited to generate gold nanocluster arrays that eliminates the high cost associated with conventional nanofabrication techniques while at the same time maintaining a high level of sensitivity. Together with the ease in transferring a pattern onto the flexible tape, the new design represents a significant advancement in on-field analytics making flexible-SERS much more accessible in terms of cost and performance. In addition, the flexible substrates are transparent and allow the direct positioning of the SERS-tape onto the object under investigation for efficient analyte collection and detection. The technique demonstrated the possibility of high detection limits, is quantitative and has fairly low signal variability across the substrate surface (<10%).
2. Experimental
2.1. Materials
Quartz wafers were purchased from SYST Integration Pte. Ltd., Singapore. Kapton® tape was purchased from S-Chem International (S) Pte. Ltd. Polystyrene-block-poly(2-vinylpyridine) (PS-b-PVP) (57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-b-57
000-b-57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, PDI = 1.1) was purchased from Polymer Source Inc., (Montreal, Canada). 2-Propanol and m-xylene were obtained as anhydrous solvents with purity > 99% from Sigma-Aldrich Pte Ltd. Crystal violet (Sigma), sodium citrate (Sigma) and hydrogen tetrachloroaurate(III) trihydrate (HAuCl4·3H2O) (99.9%, Aldrich) were used as received. Point Probe Plus silicon tips for imaging in tapping mode with atomic force microscopy were purchased from Nanosensors (Neuchatel, Switzerland).
000 g mol−1, PDI = 1.1) was purchased from Polymer Source Inc., (Montreal, Canada). 2-Propanol and m-xylene were obtained as anhydrous solvents with purity > 99% from Sigma-Aldrich Pte Ltd. Crystal violet (Sigma), sodium citrate (Sigma) and hydrogen tetrachloroaurate(III) trihydrate (HAuCl4·3H2O) (99.9%, Aldrich) were used as received. Point Probe Plus silicon tips for imaging in tapping mode with atomic force microscopy were purchased from Nanosensors (Neuchatel, Switzerland).
2.2. Methods
The quartz wafers were diced into 10 mm × 10 mm size chips. They were cleaned in acetone and 2-propanol for 10 min and finally exposed to UV/ozone to remove organic contaminants (SAMCO UV-1, SAMCO Inc., Kyoto, Japan) for 10 minutes. The array of Au nanoparticles (AuNPs) was fabricated as described elsewhere.26 In short, reverse micelle of polystyrene-block-poly(2-vinylpyridine) (PS-b-PVP) of 0.5% (w/w) concentration in m-xylene were self-assembled on the quartz surface by spin-coating where PVP forms the core and PS forms the continuous layer. This resulted in the regular arrangement of the polymer features with pitch of ∼75 nm. This was followed by immersion of the polymer coated substrates in aqueous suspension of citrate ion stabilized pre-synthesized AuNPs for 2 hours. This initiates the directed electrostatic self-assembly of the negatively charged AuNPs on to the positively charged pyridyl units of the PVP core reverse micelles. The polymer template was then removed by exposing them to O2 plasma reactive ion etch (Oxford Plasmalab 100, Oxfordshire, UK) for 10 min (30 W, 65 mTorr, 20 sccm O2) to complete the nanocluster array fabrication. The AuNP colloidal solution was synthesized according to a procedure reported in the literature.33 The pattern geometry such as the mean heights, width and pitch of the arrays were characterized using tapping mode AFM (Dimension Icon, Bruker Corporation, CA, USA). The plan view of the gold nanoparticles dispensed on a Cu grid was obtained using transmission electron microscopy performed using Philips CM300 TEM operating at 300 kV. The gold nano cluster arrays were then transferred onto the adhesive coated Kapton tape by sticking the latter to the quartz chip followed by gentle pressing and peeling off. The SERS spectra were recorded using a uRaman system (Technospex, Singapore) equipped with a Nikon microscope (Nikon Ci-L), a TE cooled CCD detector, and an excitation laser of 785 nm. The laser was coupled through a 20× objective with numerical aperture of 0.7 for exciting the sample as well as collecting the Raman signals. Prior to each Raman experiment, the instrument was calibrated using the Raman signal from a silicon standard centered at 521 cm−1. The analyte solution was dispensed onto the SERS substrate and a glass coverslip is placed over it. In order to confirm the consistency of the SERS-tape, measurements were performed at 8–10 random locations on each sample. The laser power was set at 38.7 mW and acquisition time of 5 s was kept constant for all experiments.3. Results and discussion
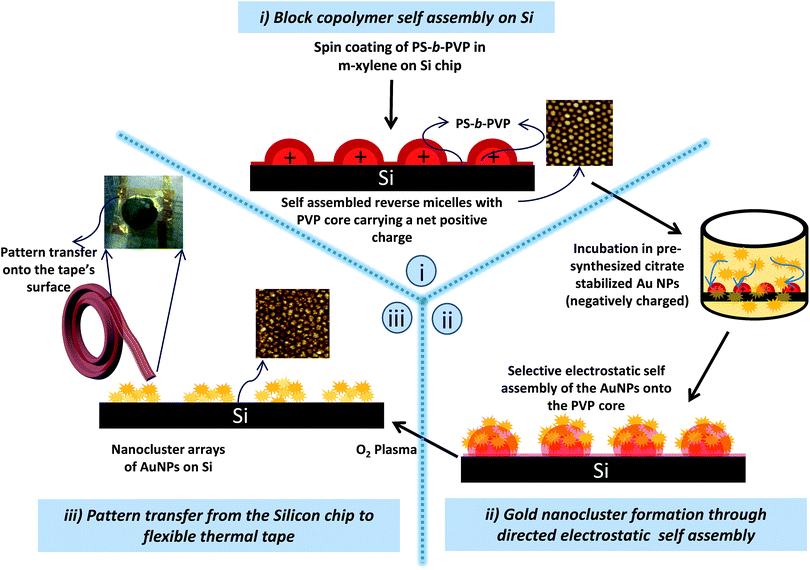
The block-copolymer self-assembly process is a versatile bottom-up approach capable of achieving patterned arrays at the wafer-level. A detailed illustration of the self-assembly process towards the fabrication of flexible substrates starting from gold nanoclusters on flat chips is shown in Fig. 1. The PVP forms the core and is responsible for pattern formation while the PS block of the BCP forms a thin continuous layer. The latter prevents any random deposition when the self-assembled patterns are introduced to a solution of negatively charges pre-formed gold nanoparticles. The PVP core that contains the pyridyl units becomes positively charged upon protonation and selectively guides the electrostatic self-assembly of the AuNPs (pH 5.8) onto its surface thus forming islets of AuNP clusters.17 After the selective deposition, the BCP template is removed by exposing the arrays to oxygen plasma etch for 10 min resulting in gold nanocluster arrays. Fig. 2 shows the as spin-coated BCP arrays of PS-b-PVP with a height of ∼23 nm and diameter of ∼55 nm that acted as the template to guide the electrostatic self-assembly of pre-formed AuNPs of size 11.5 nm (inset in Fig. 2b) in order to form the gold nanocluster arrays. A histogram showing the distribution in the size of the nanoparticles can be found in Fig. S1 of the ESI.†We have reported the SERS performances of the resulting arrays on conventional rigid substrates for concentrations up to picomolar.17,34 Nevertheless, the need to have SERS substrates that allow more versatility for practical applications and on curved non-planar surfaces where flat chips fail to establish their utility calls for the need to have flexible substrates. The latter remain highly useful for specific tasks that involve forensic analysis, picking chemicals from surfaces inaccessible to flat SERS chips and in the food processing industry to check for contamination. A Kapton tape is then attached to the flat chip on which the AuNP clusters were formed and pressed down gently. The tape was eventually peeled off and this results in the transfer of the entire SERS-active assembly from the chip to the tape. Quartz, being transparent, had been the support on which the SERS-sensitive layers were built in order to demonstrate the complete transfer of the SERS-active components onto the tape without leaving behind any residue, although, Si can also be employed. An AFM performed on the quartz chip after the pattern transformation shows no signs of residual patterns of gold and with the surface exhibiting a roughness of <1 nm (Fig. S2 of the ESI†).
The ‘stick and peel’ technique as depicted in Fig. 3A to transfer the gold nanocluster patterns from the chip to the tape overcome several issues that are associated with direct fabrication of metal cluster patterns on tape. The surface of the tape is rough and its not compatible with standard fabrication techniques. Furthermore, the micelles from the solution did not self-assemble, owing to the incompatibility in the surface energy of the tape to the apolar solvent. On the other hand, the ‘stick and peel’ technique has ensured complete pattern-transfer as evident from the clean quartz chip after transfer (Fig. S2†). More importantly, the gold nanoclusters were preserved after transferring to the tape. This was confirmed by characterizing the optical properties of the nano cluster arrays using transmittance measurements performed with a micro spectrophotometer (CRAIC Technologies, CA, US) that enabled measurement areas of 23 μm × 23 μm. This provides more qualitative information on the pattern integrity at a highly localized space on the substrate than a scanning electron microscopy (SEM) which often burns the polymer. Moreover, a coating of the SERS-active tape with a thin metal layer to counter the charging in SEM affects the observation of the actual pattern. Nevertheless, an attempt to image the gold nanoclusters (without additional coating) on the tape was made using low energy SEM that revealed the nanoclusters were indeed intact after transfer (Fig. S3†). It is reported earlier, that the resonance wavelength is known to be sensitive towards the arrangement and packing density of the AuNPs.17 The transmittance spectra for both the SERS-chip and tape exhibit a single broad peak around 730 nm (Fig. 3B), with no evidence of shift associated with any plausible change in the pattern-arrangement. The ’stick and peel’ method provides a route to precisely control AuNPs arrangement on flexible substrates to yield high SERS enhancement; this is not easily achievable by direct fabrication.
While flexible SERS substrates are advantageous in terms of its utility in real world applications, they usually suffer in terms of their robustness, compared to traditional support like silicon and glass. On the other hand, Kapton® polyimide tape with silicon adhesive as the support material is known to offer good chemical, mechanical and thermal stability. Chemically stability is vital as SERS substrates are exposed to harsh and corrosive solvents that contains the analyte of interest. A support that is susceptible to solvent exposure will result in destruction of plasmonic hot spots and losing its sensitivity for detection. The chemical stability of our SERS-tape was evaluated by immersion of the entire substrate into various solvents, i.e. water, hexane (apolar) and ethanol (polar) for 15 min. There was no observable change in the plasmonic resonance wavelength of the gold nano clusters after immersion, suggesting that (1) the tape is able to withstand the treatment in solvents, and (2) the adhesive layer on the tape is stable and maintained the arrangement of the gold nano clusters (Fig. 3C).
Temperature stability of the support is necessary as the SERS substrates can be exposed to high temperatures which arise when the laser is focused at the same spot for prolong times. Paper based SERS substrates tend to burn easily and result in fluctuation in Raman intensity, making quantification studies difficult. This issue was not observed on our SERS-tape as the Kapton support is stable up to 400 °C. In addition, the SERS-tape has low background signal, which facilitates ultra-low concentration detection (Fig. S4†).
An effective SERS substrate needs to have shelf time stability for its high SERS enhancement. Degradation in SERS enhancement may arise due to oxidation or change in the physical arrangement of the metallic nanostructures or deposition of contaminants from the ambient environment onto the substrate which prevents absorption of the analyte molecules. Any of these occurrences will result in change in the optical properties of the gold nano clusters, which will be readily picked up by micro spectrophotometer measurements. The prepared SERS-tapes were kept under normal laboratory storage conditions for one month and the transmission spectra were collected at various time points. There was no observable change in the spectra over a period of one month suggesting that there was no change in the adhesive characteristics and variation in the charges on the tape surface, which kept the gold nano clusters intact (Fig. 3D).
The SERS-tapes were assessed for its detection sensitivity using, crystal violet (CV), a test analyte that physisorbed to the substrate via non-covalent interaction. CV is non-resonant with the excitation wavelength of 785 nm. The Raman characteristics bands of CV were clearly shown for all concentrations from 1 mM down to 1 μM (Fig. 4A). A control experiment with bare tape (no gold nanoclusters) showed no enhancement. As the polyimide based Kapton tape has excellent optical transparency, we hypothesised that the Raman signal can be collected by illuminating the laser from the backside of the tape. The SERS intensities collected through the backside for the same concentration of CV is shown in Fig. 4B, indicating that the intensities are very similar to those obtain by excitation from the front side. This suggest that the SERS-tape is indeed highly transparent to light around 785 nm and can be used for direct collection and analysis of analyte on irregular surface by pasting the SERS-tape over it. This is especially useful for detection and quantification of contaminants on the surface of an arbitrary shaped object. In addition, SERS-tape has a secondary function of serving as an adhesive, which facilitates easy attachment to the object of interest.
To demonstrate this, a curved (non-planar) object, viz., a rod of diameter ∼8 mm was locally contaminated with 50 μM CV in water (Fig. 4C). The SERS tape was then wrapped around the object, with the plasmonic gold structures in contact with the analyte and the Raman signal was collected by excitation of plasmons from the back side mode. The most intense peak of CV at 1172 cm−1 was used for comparison of the signal obtained at different concentration. The enhanced intensity fell in the region between the intensities observed for 100 μM and 10 μM concentrations of CV (Fig. 4D). Although, many reports have demonstrated the ability to detect trace quantities of analytes, they suffer from substrate incompatibility towards harsh solvents that corrode the supporting substrates (paper based substrates), and thereby limiting the substrate’s range of applications. It is thus clear that the SERS-tape is versatile in its utility, stable and promises huge potential for large scale fabrication. Since, the methodology starts with the BCP self-assembly, the process is feasible at a wafer level with assured plausibility in the pattern-transfer of the plasmonic features to a tape of similar dimension. As an extension, the flexible SERS-tape was tested for its performance using 1-thionaphthol in n-hexane and potassium dichromate in water and still can be extended to a range of other chemicals such as melamine and carbaryl (pesticide) for detecting adulteration or contamination in food products.
4. Conclusions
Recent advances in portable Raman instrumentation have dramatically increased their application for on-field analysis. With this development, we can no longer be tied to flat and rigid SERS substrates. What is required are flexible and transparent SERS substrates which can fit onto irregular shaped samples and one that facilitates direct in situ measurement of the analyte without an additional extraction step. In this paper, we report the fabrication of gold nanoclusters arrays on flexible tapes for SERS applications. The SERS-tape does not succumb easily to harsh environments by virtue of the substrate property and nanoclusters integrity and thereby enabling its usage to a wide range of operating conditions. The gold nanoclusters created on Kapton tape extend their adaptability to wide range of analytes in various polar and apolar solvents. Moreover, with wafer-level processing, large area SERS-tapes are easy to fabricate with the simple ‘stick and peel’ technique (yield is dependent on the wafer size) to generate large quantities of flexible SERS-tapes within short period of time. This offers excellent opportunities to further pursue the development of innovative SERS substrates for laboratory and industrial applications on a large scale.Acknowledgements
Funding received from Exploit Technologies Pte Ltd (ETPL), A*STAR is gratefully acknowledged. The authors also thank SnFPC, IMRE, for providing fabrication and characterization facilities.References
- B. Liu, P. Zhou, X. Liu, X. Sun, H. Li and M. Lin, Food Bioprocess Technol., 2013, 6, 710–718 CrossRef CAS.
- F. Deiss, N. Sojic, D. J. White and P. R. Stoddart, Anal. Bioanal. Chem., 2010, 396, 53–71 CrossRef CAS PubMed.
- G. F. S. Andrade, M. Fan and A. G. Brolo, Biosens. Bioelectron., 2010, 25, 2270–2275 CrossRef CAS PubMed.
- M. Fan, G. F. S. Andrade and A. G. Brolo, Anal. Chim. Acta, 2011, 693, 7–25 CrossRef CAS PubMed.
- L. Scarabelli, M. Coronado-Puchau, J. J. Giner-Casares, J. Langer and L. M. Liz-Marzán, ACS Nano, 2014, 8, 5833–5842 CrossRef CAS PubMed.
- P. Guo, D. Sikdar, X. Huang, K. J. Si, W. Xiong, S. Gong, L. W. Yap, M. Premaratne and W. Cheng, Nanoscale, 2015, 7, 2862–2868 RSC.
- M. R. Langille, M. L. Personick, J. Zhang and C. A. Mirkin, J. Am. Chem. Soc., 2012, 134, 14542–14554 CrossRef CAS PubMed.
- A. J. Chung, Y. S. Huh and D. Erickson, Nanoscale, 2011, 3, 2903–2908 RSC.
- X.-M. Lin, Y. Cui, Y.-H. Xu, B. Ren and Z.-Q. Tian, Anal. Bioanal. Chem., 2009, 394, 1729–1745 CrossRef CAS PubMed.
- Y. Weisheng, W. Zhihong, Y. Yang, C. Longqing, S. Ahad, W. Kimchong and W. Xianbin, J. Micromech. Microeng., 2012, 22, 125007 CrossRef.
- W.-Y. Zhang, X.-Z. Xiao, C. Lv, J. Zhao, G. Wang, X. Gu, R. Zhang, B.-B. Xu, D.-D. Zhang, A.-W. Li, Y.-L. Zhang and H.-B. Sun, Macromol. Res., 2013, 21, 306–310 CrossRef CAS.
- T. Siegfried, M. Kind, A. Terfort, O. J. F. Martin, M. Zharnikov, N. Ballav and H. Sigg, J. Raman Spectrosc., 2013, 44, 170–175 CrossRef CAS PubMed.
- J. Li, C. Chen, H. Jans, X. Xu, N. Verellen, I. Vos, Y. Okumura, V. V. Moshchalkov, L. Lagae and P. Van Dorpe, Nanoscale, 2014, 6, 12391–12396 RSC.
- A. Martin, J. J. Wang and D. Iacopino, RSC Adv., 2014, 4, 20038–20043 RSC.
- L.-B. Zhong, J. Yin, Y.-M. Zheng, Q. Liu, X.-X. Cheng and F.-H. Luo, Anal. Chem., 2014, 86, 6262–6267 CrossRef CAS PubMed.
- L. Polavarapu and L. M. Liz-Marzan, Phys. Chem. Chem. Phys., 2013, 15, 5288–5300 RSC.
- F. L. Yap, P. Thoniyot, S. Krishnan and S. Krishnamoorthy, ACS Nano, 2012, 6, 2056–2070 CrossRef CAS PubMed.
- J. F. Betz, W. W. Yu, Y. Cheng, I. M. White and G. W. Rubloff, Phys. Chem. Chem. Phys., 2014, 16, 2224–2239 RSC.
- D. He, B. Hu, Q.-F. Yao, K. Wang and S.-H. Yu, ACS Nano, 2009, 3, 3993–4002 CrossRef CAS PubMed.
- P. M. Fierro-Mercado and S. P. Hernández-Rivera, Int. J. Spectrosc., 2012, 2012, 716527 Search PubMed.
- M. Fan, Z. Zhang, J. Hu, F. Cheng, C. Wang, C. Tang, J. Lin, A. G. Brolo and H. Zhan, Mater. Lett., 2014, 133, 57–59 CrossRef CAS PubMed.
- C. H. Lee, L. Tian and S. Singamaneni, ACS Appl. Mater. Interfaces, 2010, 2, 3429–3435 CAS.
- W. W. Yu and I. M. White, Analyst, 2013, 138, 1020–1025 RSC.
- C. Zhenyi, D. Zhangmin, C. Na, L. Shupeng, P. Fufei, L. Bo and W. Tingyun, IEEE Photonics Technol. Lett., 2014, 26, 777–780 CrossRef.
- H. Chen, F. Tian, J. Chi, J. Kanka and H. Du, Opt. Lett., 2014, 39, 5822–5825 CrossRef CAS PubMed.
- V. Suresh, S. Madapusi and S. Krishnamoorthy, ACS Nano, 2013, 7, 7513–7523 CrossRef CAS PubMed.
- S. Park, D. H. Lee, J. Xu, B. Kim, S. W. Hong, U. Jeong, T. Xu and T. P. Russell, Science, 2009, 323, 1030–1033 CrossRef CAS PubMed.
- V. Suresh, M. S. Huang, M. P. Srinivasan and S. Krishnamoorthy, J. Mater. Chem., 2012, 22, 21871–21877 RSC.
- V. Suresh, M. S. Huang, M. P. Srinivasan and S. Krishnamoorthy, ACS Appl. Mater. Interfaces, 2013, 5, 5727–5732 CAS.
- V. Suresh, D. Y. Kusuma, P. S. Lee, F. L. Yap, M. P. Srinivasan and S. Krishnamoorthy, ACS Appl. Mater. Interfaces, 2015, 7, 279–286 CAS.
- S. Krishnamoorthy, K. K. Manipaddy and F. L. Yap, Adv. Funct. Mater., 2011, 21, 1102–1112 CrossRef CAS PubMed.
- F. L. Yap and S. Krishnamoorthy, J. Mater. Chem., 2010, 20, 10211–10216 RSC.
- K. C. Grabar, R. G. Freeman, M. B. Hommer and M. J. Natan, Anal. Chem., 1995, 67, 735–743 CrossRef CAS.
- S. Dinda, F. L. Yap, V. Suresh, R. K. Gupta, D. Das and S. Krishnamoorthy, Aust. J. Chem., 2013, 66, 1034–1038 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: AFM of the chip after pattern-transfer, histogram of nanoparticles, SEM of the SERS tape and background Raman signal of the SERS-tape. See DOI: 10.1039/c5ra09934j |
| This journal is © The Royal Society of Chemistry 2015 |