Efficient catalyst for tandem solvent free enantioselective Knoevenagel-formal [3+3] cycloaddition and Knoevenagel-hetero-Diels–Alder reactions†
Sylvia Fernandesa,
P. Rajakannub and
Sujata V. Bhat*a
aLaboratory for Advanced Research in Natural and Synthetic Chemistry, V. G Vaze College, Mumbai University, Mithagar Road, Mulund East, Mumbai 400 081, India. E-mail: Sujata8b@gmail.com
bDepartment of Chemistry, Indian Institute of Technology, Powai, Mumbai 400 076, India
First published on 29th July 2015
Abstract
In this study a highly efficient catalyst has been observed for tandem solvent free enantioselective Knoevenagel-formal [3+3] cycloaddition and Knoevenagel-hetero-Diels–Alder reactions. Thus, the synthesis of bicyclic tetrahydro-2H-chromen-5(6H)-one and tricyclic octahydro-2H-benzo[c]-chromen-1(6H)-one derivatives with enantioselectivity up to ee 99% has been achieved in the presence of a chiral Lewis acid assisted Brønsted acid (LBA), titanium-isopropoxy-(S)-BINOLate under solvent free conditions. The stereochemistry of the tricyclic product 10 has been further supported by single crystal X-ray analysis. This domino powerful strategy combines both the economic and environmental aspects of organic chemistry, which are necessary for academic and industrial applications.
Introduction
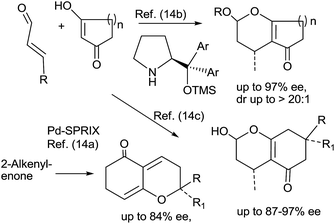
Several bioactive natural products contain the tetrahydro-benzopyran moiety,1 some examples being forskolin,2 hongoquercin A,3a zanthosimuline,3b chromazonarol,3c Δ9-tetrahydrocannabinol (THC),4 cannabichromene (CBC).5a etc. Asymmetric catalysis of organic reactions to provide enantiomerically enriched products is of central importance to modern synthetic and pharmaceutical chemistry. Therefore, the need for truly efficient and practical asymmetric synthesis has been one of the greatest challenges for synthetic chemists.6A common approach for the synthesis of bicyclic tetrahydro-2H-chromen-5(6H)-one and tricyclic octahydro-2H-benzo[c]-chromen-1(6H)-one rings utilizes Knoevenagel-[3+3] cycloaddition and Knoevenagel-hetero-Diels–Alder reactions. Several catalysts have been reported for these reactions.7–13 In previous reports, the reactions between 1,3-dicarbonyls (2 or 3) and olefinic aldehydes 4a or 4b were evaluated in the presence of catalysts such as InCl3,7 EDDA,8p-TSA,9 BF3OEt2,9 TiCl4,9 In(OTf)3,9 phosphoric acid,10 EDDA/ZnCl2,11 water,12 NaOMe/MeOH13a etc. A recent report utilizes a new hollow-structured ZIF-8-H nanosphere as a catalyst for [3+3] cycloaddition reactions.13b
In previous reports, enantioselective cyclization of 2-alkenyl-1,3-diones promoted by Pd-SPRIX catalyst was evaluated, where isomeric 2,2-dialkyl-6,7-dihydro-2H-chromen-5(3H)-ones were formed in ee up to 88%.14a Similarly, the enantioselective addition of conjugated aldehyde to 1,3-cyclohexadione and cyclopentadione in the presence of organo-catalyst (trimethylsilyl-1,1-diaryl-prolinol) has been achieved, which yielded 4-substituted-2-hydroxy or 2-acyloxy-chromenones14b,c in ee up to 97% (Scheme 1).
We report herein a highly efficient tandem solvent free enantioselective Knoevenagel-formal [3+3] cycloaddition and Knoevenagel-hetero-Diels–Alder reactions in the presence of the chiral LBA titanium-isopropoxy-(S)-BINOLate (1) (Fig. 1).
Thus, the synthesis of bicyclic tetrahydro-2H-chromen-5(6H)-one and tricyclic octahydro-2H-benzo[c]-chromen-1(6H)-one derivatives with enantioselectivity up to 98.8% has been achieved in the presence of chiral LBA, under a solvent free condition (Scheme 2, Table 1).
 | ||
| Scheme 2 Stereoselective reactions of cyclohexa-1,3-dienones with aldehydes in the presence of [(S)-(−)-BINOLate]2Ti2(O-i-Pr)4 (chiral LBA, 1). | ||
Results and discussion
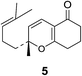
The chiral catalyst 1 was prepared according to the reported procedure.15 Initially, the reaction of 1,3-cyclohexadienone (2) and citral (4a) in CH2Cl2 at 0 °C under N2 atmosphere was attempted, which went to completion in 1/2 h, with 79% yield. A similar reaction of 1,3-cyclohexadienone (2) and citral (4a) in the presence of chiral catalyst 1 under solvent free condition gave similar yield in the same duration of time. Hence we carried further reactions under solvent free condition.The reaction between α,β-unsaturated aldehyde such as citral (4a) (17.90 mmol) and 1,3-cyclohexadienone (2) or dimedone (3) (17.90 mmol) was achieved in the presence of 1 (3 mol%) at 0 °C under solvent free condition. The reaction was monitored by GC and TLC analyses and was completed within 1/2 h to give the bicyclic tetrahydro-2H-chromen-5(6H)-one derivatives 5 (80% yield and 99% ee) and 8 (77% yield and 89% ee) respectively (Table 1, entries 1 and 4).
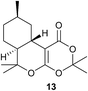
Additional reactions of 1,3-dicarbonyl compounds 2 or 3 with terpenic olefinic aldehydes such as (R)-citronellal (4b), (R)-melonal (4c) were also evaluated on the same reaction scale, which yielded tricyclic octahydro-2H-benzo[c]-chromen-1(6H)-one derivatives 6, 7, 9 and 10 respectively in ∼77% yields and ee in the range of 88–98% (Table 1, entries 2, 3, 5, 6). Similarly, the reaction of cyclohexa-1,3-dione (2) with conjugated aldehyde (E);7-formyl-3-methyl-oct-6-enyl acetate (4d) yielded bicyclic chromenone adduct 11 (75% yield and 98% ee). The reaction of Meldrum's acid (12) with (R)-citronellal (4b) gave tricyclic [1,3]dioxino[4,5-c]isochromen-1(6H)-one derivative 13. The reaction of cyclopenta-1,3-dione with (R)-citronellal (4b) did not yield tricyclic product. Apparently Knoevenagel adduct intermediate in case of cyclopenta-1,3-dione does not undergo hetero-Diels–Alder reaction under present reaction condition.
The required aldehydes (R)-melonal (4c) and (R)-(E)-7-formyl-3-methyl-oct-6-enyl acetate (4d) were synthesised with minor modifications of reported procedures.16
The structures of compounds 5–11, 14 and 15 were identified with IR, 1H-NMR, 13C-NMR, GC/MS and HRMS data. In 1H-NMR spectrum, the olefinic protons of 5 absorbed at δ 6.45 (d, J = 10.12 Hz, 1H), 5.18 (d, J = 10.12 Hz, 1H). 13C-NMR spectrum of compound 5 showed peaks at δ 194.9 and 172.1 due to C-5 carbonyl and C-8a enol carbon respectively. The olefinic carbons absorbed at δ 131.9, 123.6, 121.6, 116.4, 110.3 and ethereal carbon at δ 82.4. 1H-NMR compound 6 showed absence of olefinic protons. The 13C-NMR spectrum of compound 6 showed peaks at δ 197.8 and 170.5 due to carbonyl and enol carbon respectively. The 1H-NMR and 13C-NMR of remaining molecules were also in agreement with their structures.
The enantiomeric excess (ee%) of compounds 5–11, 14, 15 was obtained in GC analysis using chiral Beta-Dex column. High stereoselectivity was observed, ee% ranging from 88.1–98.8%, for both bicyclic (5, 8, 11) and tricyclic (6, 7, 9, 10) molecules (Table 1). The structure and stereochemistry of tricyclic compound 10 was further supported by single crystal X-ray analysis,17–19 which is shown in Fig. 2. It is noteworthy, that the stereochemistry at ring junction positions 3a and 9b of analogue 10 is opposite to that of natural (R,R)-THC.4 In view of strong analgesic activity of (S,S)-daxanabinol5b the present report has more significance.
The 2-(R)-stereochemistry of 7,8-dihydro-2H-chromen-5(6H)-one derivatives 5, 8 and 11 was assigned based on (−)-optical rotation and CD spectra.20,21
The molecules 7, 10 and 11 are novel to the best of our knowledge.
The probable mechanism for stereoselective formation of (2R)-5,6,7,8-tetrahydro-5-oxo-2H-chromene derivatives is shown in Fig. 3. In intermediate A the bulky substituent (R–) in aldehyde prefers equatorial position, which is converted to condensation product intermediate B. The elimination of water molecule leads to intermediate C, subsequent attack by isopropanol releases (2R)-5,6,7,8-tetrahydro-5-oxo-2H-chromene derivative and the catalyst 1 is regenerated. Similar mechanism for stereospecific formation of tricyclic octahydro-6,6,9-trimethyl-2H-benzo[c]-chromen-1(6H)-one derivatives is given in Fig. 4. The aldol condensation of intermediate A leads to intermediate B, which undergoes dehydration to give C, the hetero-Diels–Alder reaction, subsequent attack by isopropanol and release of tricyclic octahydro-6,6,9-trimethyl-2H-benzo[c]-chromen-1(6H)-one derivative regenerates the catalyst 1.
 | ||
| Fig. 3 The probable mechanism of formation of (2R)-5,6,7,8-tetrahydro-5-oxo-2H-chromene derivatives. | ||
 | ||
| Fig. 4 The probable mechanism of formation of octahydro-6,6,9-trimethyl-2H-benzo[c]-chromen-1(6H)-one derivatives. | ||
The present catalytic system provides an attractive protocol to various optically active derivatives of bicyclic chromen-5(6H)-one and tricyclic benzo[c]-chromen-1(6H)-one in terms of the following features: (i) the catalyst is inexpensive and easily available; (ii) the protocol has a broad scope of substrates; (iii) the reactions show high enantioselectivity; (iv)short reaction time, reactions are completed within 1/2 h; (v) the reaction is environmentally benign because of solvent-free condition; and (vi) low catalyst loading (3 mol%) is sufficient to achieve high yield and optical purity of the products. We hope our findings in this research will stimulate further work on practical asymmetric synthesis of more molecules of biological and pharmacological importance.
Conclusions
In summary, highly efficient catalyst has been observed for tandem solvent free enantioselective Knoevenagel-formal [3+3] cycloaddition and Knoevenagel-hetero-Diels–Alder reactions.Thus, the synthesis of bicyclic tetrahydro-2H-chromen-5(6H)-one and tricyclic octahydro-2H-benzo[c]-chromen-1(6H)-one derivatives with enantioselectivity up to ee 99% has been achieved in the presence of titanium-isopropoxy-(S)-BINOLate under a solvent free condition. The stereochemistry of tricyclic product 10 has been further supported by single crystal X-ray analysis. The methodology reported herein can be used for the synthesis of various natural and non-natural molecules with chromenone moiety. This one-pot powerful strategy combines both the economic and environmental aspects of organic chemistry which are necessary for academic and industrial applications.
Experimental
General procedures
The monitoring of reaction and checking the purity of the products were done using pre-coated silica gel plates (Merck) and visualization using anisaldehyde/H2SO4 reagent. FT-IR spectra were recorded on a Perkin-Elmer Spectrum One spectrometer. 1H-NMR spectra were recorded on a Varian spectrometer at 400 MHz and 13C-NMR at 100 MHz; δ in ppm rel. to Me4Si as internal standard, J in Hz. Multiplicities is reported as follows: s = singlet, d = doublet, dd = doublets of doublet, t = triplet, q = quartet, m = multiplet, brs = broad singlet. GC-MS was carried on an Agilent instrument, where GC-6890 was coupled with a mass spectrometer MS-5973 N with quadrapole mass detector, using a HP-5 (5% phenyl methyl siloxane) column. Electrospray ionization and a TOF mass analyser were used for HRMS measurements. The compounds (5–11) showed the required m/z: (M+) values in HR-MS. Enantiomeric excess (ee%) was obtained in GC analysis using chiral Beta Dex 120 column (30 m × 0.25 μm × 0.25 mm). Silica gel (100–200 mesh), which was used for column chromatography (CC) was activated by heating at 120° for 4 h. All asymmetric reactions were performed under inert atmosphere and at 0 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5 mL) and filtered. The filtrate was evaporated and the residue was purified by silica gel column chromatography to afford the pure products.
1, 5 mL) and filtered. The filtrate was evaporated and the residue was purified by silica gel column chromatography to afford the pure products.
(R)-2-Methyl-2-(4-methylpent-3-enyl)-7,8-dihydro-2H-chromen-5(6H)-one (5). Yellow oil; [α]D = −65.2° (c = 1 in MeOH), chiral GC analysis: 33.79 (major), 33.19 (minor) min, ee 99%; IR (CHCl3, cm−1): 2939, 1647, 1603, 1428, 1381, 1333, 1285, 1257, 1184, 1111, 1081, 967, 864, 787; 1H-NMR (CDCl3, 400 MHz, TMS): δ (PPM) 6.45 (d, J = 10.12 Hz, 1H), 5.18 (d, J = 10.12 Hz, 1H), 5.09–5.07 (m, 1H), 2.42–2.39 (m, 4H), 2.01–1.94 (m, 4H), 1.74–1.71 (m, 1H), 1.68 (s, 3H), 1.59 (s, 3H),1.68–1.53 (m, 1H), 1.36 (s, 3H); 13C-NMR (150 MHz, TMS): δ (PPM) 194.9, 172.1, 131.9, 123.6, 121.6, 116.4, 110.3, 82.4, 41.7, 36.4, 28.6, 27.4, 25.7, 22.5, 20.6, 17.6; GC-MS (m/z): 246, 231, 213, 203, 190, 175, 163, 155, 147, 135, 122, 107, 99, 91, 77, 69, 55, 41; HRMS (EI+) calcd for C16H22O2 246.1620, found m/z 246.1622.
Synthesis of (±)-2-methyl-2-(4-methylpent-3-enyl)-7,8-dihydro-2H-chromen-5(6H)-one (5) using ethylenediamine diacetate as catalyst. To a mixture of 1,3-cyclohexadione, 2 (2 g, 17.9 mmol) and citral 4a, (2.73 g,17.9 mmol) was added catalyst EDDA (5 mol%) and stirred at room temperature for 1 h. After completion of the reaction, the reaction mixture was purified by column chromatography using n-hexane/EtOAc (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to provide 5 (3.31 g, 75.4%) as a yellow oil. [α]D = 0.
1) as eluent to provide 5 (3.31 g, 75.4%) as a yellow oil. [α]D = 0.
(6aS,9R,10aS)-6,6,9-trimethyl-2,3,4,6,6a,7,8,9,10,10a-dehydro-1H-benzo[c]chromen-1-one (6). Colourless solid; [α]D = −100.1° (c = 1 in MeOH), chiral GC analysis 36.61 min ((major), 37.78 min (minor), ee% 97); IR (CHCl3, cm−1): 2920, 2847, 1649, 1598, 1447, 1379, 1273, 1252, 1192, 1128, 999, 850, 775, 693; 1H-NMR (CDCl3, 400 MHz, TMS): δ (PPM) 2.80–2.77 (ddt, J = 11.5, 3, 1.7 Hz, 1H), 2.43–2.18 (m, 4H), 2.13–2.06 (dt, J = 11.08, 2.2 Hz, 1H), 1.92–1.86 (m, 2H), 1.86–1.78 (m, 1H), 1.78–1.72 (m, 1H), 1.61–1.50 (m, 1H), 1.33 (s, 3H), 1.29–1.23 (td, J = 11.3, 2.6 Hz, 1H), 1.05 (s, 3H), 1.12–0.95 (m, 2H), 0.91–0.89 (d, J = 6.6 Hz, 3H), 0.49 (q, J = 11.5 Hz, 1H); 13C-NMR (100 MHz, TMS): δ (PPM) 197.8, 170.5, 114.7, 80.3, 48.6, 38.8, 37.5, 35.5, 33.6, 32.3, 29.6, 27.5, 27.2, 22.5, 20.2, 19.4; GCMS (m/z): 248, 233, 219, 205, 192, 177, 163, 150, 137, 123, 109, 95, 81, 68, 55, 41; HRMS (EI+) calcd for C16H24O2 248.1776, found m/z 248.1774.
(1R,3aS,9bS)-1,4,4-Trimethyl-1,2,3,3a,4,7,8,9b-octahydrocyclo-penta[c]chromen-6(6H)-one (7). Colourless liquid. [α]D = −86.6° (c = 1, MeOH); chiral GC analysis 34.23 (major), 33.88 (minor) min, (ee% 88); IR (CHCl3, cm−1): 2945, 2869, 1651, 1582, 1455, 1428, 1379, 1334, 1278, 1226, 1192, 1113, 1064, 1004, 991, 848, 629; 1H-NMR (CDCl3, 400 MHz, TMS): δ (PPM) 2.41–2.34 (dt, J = 11.4, 5 Hz, 1H), 2.35–2.30 (m, 2H), 2.29–2.26 (m, 2H), 2.04–1.98 (m, 1H), 1.91–1.87 (m, 1H), 1.86–1.81 (m, 4H), 1.72–1.55 (m, 2H), 1.41–1.39 (d, J = 6.16 Hz, 3H), 1.36 (s, 3H), 1.13 (s, 3H); 13C-NMR (100 MHz, TMS): δ (PPM) 197.5, 171.2, 115.4, 81.5, 53.5, 42.6, 37.6, 36.6, 34.4, 29.7, 28.4, 24.8, 23.4, 20.4, 20.1; GC purity: 90.0%; GCMS (m/z): 234, 219, 191, 177, 163, 151, 137, 125, 109, 91, 79, 67, 55, 41; HRMS (EI+) calcd for C15H22O2 234.1620, found m/z 234.1622.
(R)-2,7,7-Trimethyl-2-(4-methylpent-3enyl)-7,8-dihydro-2H-chromen-5(6H)-one (8). Yellow oil. [α]D = −66.2° (c = 1, MeOH); chiral GC analysis 33.86 (major), 32.39 (minor) min, (ee% 89); IR (CHCl3, cm−1): 2961, 1680, 1606, 1449, 1382, 1331, 1287, 1217, 1198, 1165, 1112, 1077, 1034, 936, 889; 1H-NMR (CDCl3, 400 MHz, TMS): δ (PPM) 6.44 (d, J = 10 Hz, 1H), 5.17 (d, J = 10 Hz, 1H), 5.11–5.07 (m, 1H), 2.29–2.23 (m, 4H), 2.08–2.01 (m, 2H), 1.76–1.69 (m, 1H), 1.67 (s, 3H), 1.57 (s, 3H), 1.56–1.54 (s, 1H), 1.37(s, 3H), 1.08 (s, 3H), 1.06 (s, 3H); 13C-NMR (100 MHz, TMS): δ (PPM) 194.4, 170.6, 131.8, 123.6, 121.4, 116.2, 109.0, 82.5, 50.3, 42.4, 41.7, 32.1, 28.6, 28.2, 27.5, 25.7, 22.5, 17.6; GCMS (m/z): 274, 259, 241, 231, 218, 205, 191, 175, 165, 157, 149, 141, 129, 121, 107, 91, 77, 69, 55, 41; HRMS (EI+) calcd for C18H26O2 274.1933, found m/z 274.1930.
(6aS,9R,10aS)-3,3,6,6,9-Pentamethyl-2,3,4,6,6a,7,8,9,10,10a-decahydro-1H-benzo[c]chromen-1-one (9). Yellow oil. [α]D = −101.5° (c = 1, MeOH); chiral GC analysis 34.33 (major), 33.78 (minor) min, (ee% 89); IR (CHCl3, cm−1): 2957, 2868, 1644, 1606, 1455, 1381, 1360, 1278, 1236, 1165, 1088, 1031, 1013, 941, 865, 665; 1H-NMR (CDCl3, 400 MHz, TMS): δ (PPM) 2.85 (ddt, J = 11.2, 3, 1.7 Hz, 1H), 2.22–2.18 (m, 4H), 2.02–2.18 (m, 1H), 1.84–1.78 (m, 1H), 1.78–1.72 (m, 1H), 1.61–1.50 (m, 1H), 1.35 (s, 3H), 1.26 (td, J = 11.2, 2.2 Hz, 1H), 1.12–0.98 (m, 2H), 1.05 (s, 3H), 1.04 (s, 3H), 1.03 (s, 3H). 0.90 (d, J = 6.5 Hz, 3H), 0.49 (q, J = 11.2 Hz, 1H);13C-NMR (100 MHz, TMS): δ (PPM) 197.9, 175.3, 168.8, 113.2, 80.4, 51.4, 48.8, 45.4, 43.3, 43.2, 38.6, 35.6, 33.5, 32.3, 31.6, 29.3, 28.1, 27.6, 27.3, 27.2, 22.5, 19.5; GCMS (m/z): 276, 261, 243, 220, 205, 192, 177, 165, 153, 134, 123, 109, 91, 81, 69, 55, 41; HRMS (EI+) calcd for C18H28O2 276.2089, found m/z 276.2091.
(1R,3aS,9bS)-1,4,4,7,7-Pentamethyl-1,2,3,3a,4,7,8,9b-octahydrocyclo-penta[c]chromen-9(6H)-one (10). Yellow oil. [α]D = −89.6° (c = 1, MeOH), chiral GC analysis 33.55 min (major), 34.23 min (minor), (ee% 99); IR (CHCl3, cm−1): 2950, 1728, 1651, 1586, 1468, 1378, 1230, 1118, 1024, 938, 860, 757; 1H-NMR (CDCl3, 400 MHz, TMS): δ (PPM) 2.29–2.10 (m, 4H), 2.08–1.99 (m, 1H), 1.99–1.91 (m, 1H), 1.89–1.81 (m, 1H), 1.73–1.57 (m, 2H), 1.44 (d, J = 6 Hz, 3H), 1.38 (s, 3H), 1.22–1.13 (m, 2H), 1.16 (s, 3H), 1.07 (s, 3H), 1.04 (s, 3H); 13C-NMR (100 MHz, TMS): δ (PPM) 198.4, 197.6, 169.4, 167.5, 114.1, 81.6, 78.3, 53.7, 51.8, 43.5, 42.5, 42.8, 36.4, 34.6, 31.7, 28.7, 28.5, 27.9, 25.0, 23.7, 20.4; GCMS (m/z): 262, 247, 219, 205, 191, 179, 165, 151, 109, 91, 83, 67, 55, 41; HRMS (EI+) calcd for C17H26O2 262.1933, found m/z 262.1930.
(R)-3-Methyl-5-((R)-3-methyl-5-oxo-5,6,7,8-tetrahydro-2H-chromen-2-yl)pentyl acetate (11). Yellow oil. [α]D = −90.5° (c = 1, MeOH), chiral GC analysis 33.88 min (major), 32.39 min (minor), (ee% 98); IR (CHCl3, cm−1): 2920, 2847, 1729, 1649, 1598, 1447, 1379, 1273, 1252, 1192, 1128, 999, 850, 775, 693; 1H-NMR (CDCl3, 400 MHz, TMS): δ (PPM) 6.21 (s, 1H), 4, 75 (dd, J = 10.8 Hz, 3.5 Hz, 1H), 4.18–4.12 (m, 4H), 2.42–2.35 (m, 4H), 2.05 (s, 3H), 1.99–1.93 (m, 2H), 1.72 (s, 3H), 1.68–1.62 (m, 1H), 1.62–1.53 (m, 2H), 1.50–1.38 (m, 2H), 0.95–0.90 (dd, J = 6.4 Hz, 3H); 13C-NMR (100 MHz, TMS): δ (PPM) 195.1, 171.2, 169.9, 126.1, 112.5, 80.9, 62.7, 36.4, 35.4, 35.1, 31.3, 30.3, 29.7, 27.9, 26.3, 20.9, 19.5, 19.1; GCMS (m/z): 306, 291, 263, 189, 163, 121, 107, 91, 77, 56, 43; HRMS (EI+) calcd for C18H26O4 306.1831 found m/z 306.1833.
(6aS,9R,10aS)-6a,7,8,9,10,10a-Hexahydro-3,3,6,6,9-pentamethyl-[1,3]dioxino[4,5-c]isochromen-1(6H)-one (13). Yellow oil. [α]D = −40.2° (c = 1, MeOH), chiral GC analysis 33.77 min (major), 32.76 min (minor), (ee% 83); IR (CHCl3, cm−1): 2925, 2855, 1736, 1726, 1620, 1455, 1390, 1280, 1155, 994, 965, 891, 731; 1H-NMR (CDCl3, 400 MHz, TMS): δ (PPM) 2.65 (dd, J = 5.6, 18.4 Hz, 1H), 2.08–2.01 (m, 1H), 1.85–0.95 (m, 6H), 1.68 (s, 3H), 1.60 (s, 3H), 1.41 (s, 3H), 1.26 (s, 3H), 0.91 (d, J = 6.5 Hz, 3H), 0.78–0.62 (m, 1H) 13C-NMR (100 MHz, TMS): δ (PPM) 171.4, 167.3, 124.4, 85.9, 47.4, 42.3, 37.2, 36.3, 32.0, 31.8, 28.7, 27.6, 25.9, 23.5, 22.3. GCMS (m/z): 280,; HRMS (EI+) calcd for C16H24O4 280.1704 found m/z 280.1703.
X-ray single crystal structure determination of molecule 10. A suitable crystal of size 0.06 × 0.18 × 0.24 mm3 was mounted on a diffractometer for unit cell determination and three dimensional intensity data collection. 800 frames in total were collected at 150 K with the exposure time of 16 s per frame. Data integration, indexing and absorption correction were followed by structure solution using the programs in a WinGX module.17 The structure was solved by direct methods (SIR-92)18 and the final refinement of the structure was carried out using full least-squares methods on F2 using SHELXL-97.19 Unit cell determination using both high and low angle reflections reveals that compound 10 crystallizes in a monoclinic P21/c space group. Non-hydrogen atoms were refined anisotropically. C–H hydrogen atoms were placed in geometrically calculated positions by using a riding model.
Acknowledgements
We are grateful to Kelkar Education Trust, Mumbai, for encouragement. We are also thankful to the Department of Chemistry and Sophisticated Analytical Instrumentation Facility, Indian Institute of Technology, Bombay, and Tata Institute of Fundamental Research, Mumbai, for NMR spectral data. We also thank Prof. R. Murugavel for the use of his Single Crystal X-ray Diffraction Facility established through a DAE-SRC outstanding investigator award.Notes and references
- (a) I. Larrosa, P. Romea and F. Urpi, Tetrahedron, 2008, 64, 2683 CrossRef CAS PubMed; (b) Y. Tang, J. Oppenheimer, Z. Song, L. You, X. Zhang and Hsung, Tetrahedron, 2006, 62, 10785 CrossRef CAS PubMed.
- (a) S. V. Bhat, B. S. Bajwa, H. Dornauer, N. J. de Souza and H.-W. Fehlhaber, Tetrahedron Lett., 1977, 18, 1669 CrossRef; (b) S. V. Bhat, B. S. Bajwa, H. Dornauer and N. J. D'Souza, J. Chem. Soc., Perkin Trans. 1, 1982, 767 RSC.
- (a) D. M. Roll, J. K. Manning and G. T. Carter, J. Antibiot., 1998, 51, 635 CrossRef CAS; (b) X. Wang and Y. R. Lee, Synthesis, 2007, 3044 CAS; (c) S. deRose, L. Minale, R. Riccio and G. Sodano, J. Chem. Soc., Perkin Trans. 1, 1976, 1408 RSC.
- (a) R. B. Mechoulam, J. Pharmacol., 2005, 146, 913 CAS; (b) E. B. Russo, Br. J. Pharmacol., 2011, 163, 1344 CrossRef CAS PubMed.
- (a) P. Pacher, S. Batkai and G. Kunos, Pharmacol. Rev., 2006, 58, 389 CrossRef CAS PubMed; (b) E. Pop, Curr. Opin. Invest. Drugs (BioMed Cent.), 2000, 1, 494 CAS.
- (a) K.-H. Wu and H.-M. Gau, J. Am. Chem. Soc., 2006, 128, 14808 CrossRef CAS PubMed; (b) J. Balsells, T. J. Davis, P. Carroll and P. J. Walsh, J. Am. Chem. Soc., 2002, 124, 10336 CrossRef CAS PubMed; (c) J. Long, J. Hu, X. Shen, B. Ji and K. Ding, J. Am. Chem. Soc., 2002, 124, 10 CrossRef CAS PubMed; (d) X. B. Yang, J. Feng, J. Zhang, N. Wang, L. Wang, J. L. Liu and X. Q. Yu, Org. Lett., 2008, 10, 1299 CrossRef CAS PubMed; (e) D. B. Biradar and H. M. Gau, Org. Lett., 2009, 11, 499 CrossRef CAS PubMed.
- Y. R. Lee, D. H. Kim, J. Shim, S. K. Kim, J. H. Park, J. S. Cha and C. S. Lee, Bull. Korean Chem. Soc., 2002, 23, 998 CrossRef CAS.
- L. Xia, H. Cai and Y. R. Lee, Org. Biomol. Chem., 2014, 12, 4386 CAS.
- A. V. Kurdyumov, N. Lin, R. P. Hsung, G. C. Gullickson, K. P. Cole, N. Sydorenko and J. J. Swidorski, Org. Lett., 2006, 8, 191 CrossRef CAS PubMed.
- J. Renaud, J. Moreau, C. Hubert, J. Batany, L. Toupet, T. Roisnel and J. Hurvois, J. Org. Chem., 2009, 74, 8963 CrossRef PubMed.
- Y. R. Lee, E. J. Jung and B. H. Park, Green Chem., 2010, 12, 2003 RSC.
- D. Kikelj and N. Zidar, Helv. Chim. Acta, 2011, 94, 0859 CrossRef PubMed.
- (a) G. Sheldrick, L. Tietze, G. Kiedrowski and K. W. Clegg, Angew. Chem., Int. Ed. Engl., 1980, 19, 134 CrossRef PubMed; (b) F. Zhang, Y. Wei, X. Wu, H. Jiang, W. Wang and H. Li, J. Am. Chem. Soc., 2014, 136, 13963 CrossRef CAS PubMed.
- (a) H. Sasai, K. Takenaka, S. C. Mohanta, M. L. Patil, C. V. L. Rao, S. Takizawa and T. Suzuki, Org. Lett., 2010, 12, 3480 CrossRef PubMed; (b) M. Rueping, E. Sugiono and E. Merino, Chem.–Eur. J., 2008, 14, 6317 CrossRef PubMed; (c) P. T. Franke, B. Richter and K. A. Jørgensen, Chem.–Eur. J., 2008, 14, 6329 CrossRef PubMed.
- P. Walsh, K. Waltz and P. Carroll, Organometallics, 2004, 23, 127–134 CrossRef.
- (a) S. Meenakshi, A. Sivaramkrishnan, R. Padmakumar, S. B. Hadimani and S. V. Bhat, Synth. Commun., 2004, 34, 4065–4076 CrossRef CAS PubMed; (b) S. Syam, M. Rane and S. V. Bhat, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2008, 47, 1308–1310 Search PubMed.
- L. J. Farrugia, WinGX, Version 1.80.05, J. Appl. Crystallogr., 1999, 32, 837.
- A. Altomare, G. Cascarano, C. Giacovazzo, A. Guagliardi, M. C. Burla, G. Polidori and M. Camalli, J. Appl. Crystallogr., 1994, 27, 435 Search PubMed.
- (a) G. M. Sheldrick, SHELXL-97, Program for crystal structure refinement, University of Göttingen, Göttingen, Germany, 1997 Search PubMed; (b) G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- N. Iwata, N. Wang, X. Yao and S. Kitanaka, J. Nat. Prod., 2004, 67, 1106–1109 CrossRef CAS PubMed.
- L. S. M. Velozo, M. J. P. Ferreira, M. I. S. Santos, D. L. Moerira, V. P. Emerenciano and M. A. C. Kaplan, Phytochemistry, 2006, 67, 492–496 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterisation data for new compounds, X-ray crystal structure details, copies of NMR, GCMS spectra and chiral GC analysis. CCDC 1060831. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra09865c |
| This journal is © The Royal Society of Chemistry 2015 |