Tuning the oxygen functional groups in reduced graphene oxide papers to enhance the electromechanical actuation†
Ganaka G. Chandrakumaraa,
Jin Shangd,
Ling Qiub,
Xi-Ya Fangc,
Frank Antolasice,
Christopher D. Eastonf,
Jingchao Songb,
Tuncay Alan*a,
Dan Li*b and
Jefferson Zhe Liu*a
aDepartment of Mechanical and Aerospace Engineering, Monash University, Clayton, Victoria 3800, Australia. E-mail: zhe.liu@monash.edu; tuncay.alan@monash.edu
bDepartment of Materials Engineering, Monash University, Clayton, Victoria 3800, Australia. E-mail: dan.li2@monash.edu
cCenter for Electron Microscopy, Monash University, Clayton, Victoria 3800, Australia
dDepartment of Chemical and Biomolecular Engineering, The University of Melbourne, Victoria 3010, Australia
eSchool of Applied Chemistry, RMIT University, Victoria 3000, Australia
fCSIRO Manufacturing Flagship, VIC 3168, Australia
First published on 24th July 2015
Abstract
The superior mechanical flexibility, mechanical strength, electrical conductivity, high specific surface area, and a special two-dimensional crystalline structure make graphene a very promising building block material for flexible electromechanical actuators. However, graphene papers have exhibited limited electromechanical actuation strain in aqueous electrolyte solution. In this paper, we show an easy approach to significantly improve the electromechanical actuation of reduced graphene oxide (rGO) papers via fine tuning the oxygen functional groups in rGO sheets, which was achieved by careful control of quantity of the reduction agent used in the chemical reduction process of graphene oxide. The actuation strains are enhanced up to 0.2% at an applied voltage of −1 V, which is more than a 2 fold increase compared to the regular pristine rGO paper. Further theoretical and experimental analyses reveal that the change of the capacitance and the stiffness of the rGO papers are two key factors responsible for the observed improvement.
1 Introduction
In the past decades, direct conversion of electrical energies into a mechanical output at small length scales has been a topic of great interest and is being investigated along a diverse range of applications, including sensors and switches,1,2 micromanipulators,3 memory chips,4 artificial muscles.5,6 An ideal actuation material should exhibit a high operation efficiency, namely a large strain output for a low power input, along with the capability of rapidly switching between different modes with a high level of precision and a long life time. Despite their high response rate, the widely used piezoelectric ceramics have drawbacks in the requirement of a high driving voltage7 and a limited strain output. In the case of polymer based materials, the limited life cycle and the low response rate8,9 are the main hurdles for their practical implementations.Recent studies show that carbon nanotube and graphene based materials are promising actuators in artificial muscles and micro/nano-electromechanical systems.10–13 Particularly, the actuation performance of graphene has been anxiously studied with expectations of revolutionising the advanced actuator systems along different actuation schemes.14–20 The studies conducted up to date show promising results. Liang et al. showed that a graphene paper (composed of well aligned multilayers of rGO sheets) was capable of achieving electromechanical strain values close to 0.064% at a low operation voltage (−1 V) due to the formation of the electrical double layer (EDL) in an aqueous electrolyte.14 They also found that introduction of nanoparticles increased interlayer spacing of the graphene sheets and allowed a better development of the EDLs, hence enhancing the actuation strain to 0.1%.14 Note that among the available techniques to maintain the interlayer distances, controlling the hydration of the corrugated rGO sheets stands out as it can realise a tunable interlayer spacing without the introduction of any alien particles nor additional synthesis steps. This technique has been developed and successfully applied to synthesis high performance graphene hydrogel papers in supercapacitors.21
In addition to the efforts of increasing the interlayer spacing of rGO sheets in the papers, another route to enhance the electromechanical strain is chemical modification. Xie et al.22,23 modified the surface of rGO papers using oxygen plasma treatment and observed an electromechanical strain value close to 0.4% in aqueous electrolyte. Ab intio simulations reported that some rGO crystal structures had a far greater actuation performance than what has been discovered. A specific ordered rGO crystal structure is predicted to have a strain of up to ∼10%6 and an irreversible strain value of 28%.24 A complete understanding of the involved mechanisms and identifying the extent of the influence by the key parameters involved would help us optimize the rGO structure to reach these predicted performance levels. However, the failure to identify the involved mechanisms and parameters has brought the development of rGO actuators and their implementation in MEMS/NEMS to an abrupt stop.
The recent advance of rGO synthesis techniques show that the oxygen content in rGO sheets can be well controlled by using different amounts of reduction agent in the synthesis process without any additional post treatment steps.25,26 He et al. found that at a weight ratio of hydrazine over graphene oxide (GO) Rw > 0.2, fine tuning of the rGO molecular structures can be achieved without causing any major disruptions to the integrity of the rGO sheets. This method can promote uniformity and integrity of the obtained rGO papers in comparison with those fabricated using the post treatment processes.
In this paper, we used three different weight ratios of hydrazine over GO in the chemical reduction process, i.e., Rw = 1.72, 0.52, and 0.34 to study the effect of oxygen functionality on electromechanical actuation in an aqueous electrolyte. The obtained products are referred to as Rw 1.72 rGO, Rw 0.52 rGO, and Rw 0.34 rGO, respectively. Using these obtained rGO solutions, three types of rGO hydrogel papers were fabricated. A dry graphene paper at Rw = 1.72 was also fabricated as a reference. We observe that electromechanical actuation of the Rw 0.52 and Rw 0.34 rGO hydrogel papers exhibit significantly enhanced actuation strains in comparison with the Rw 1.72 rGO hydrogel and dry papers. An in-depth theoretical analysis and experimental results reveal a relationship among the electromechanical strain, the electrochemical capacitance and mechanical stiffness of the papers. The knowledge gained in this study will be useful for designing of rGO paper actuators to achieve optimal electromechanical actuation performances.
2 Results and discussion
The graphene oxide dispersion was fabricated using methods reported before.27,28 In the chemical reduction process, the reduction level of the rGO sheets was controlled by managing the quantity of the hydrazine reduction agent, following the procedures reported by He et al.25 To achieve a maximum level of reduction, hydrazine (35 wt% in water) with a weight ratio value of Rw = 1.72 was mixed to GO dispersion and the chemical reduction was conducted at 100 °C for 3 h. Controlled reduction of GO dispersion was conducted at 100 °C for 2 h by using the amount of hydrazine at Rw = 0.52 and Rw = 0.34. Note that the systematic study from He et al. shows similar rGO density (mass remaining percentage) at these three levels of reduction.25 The rGO dry and hydrogel papers at specified reduction levels were synthesised via vacuum filtration following a procedure reported in ref. 21 and 29. To avoid losing water molecules, the hydrogel papers were removed from the filtration chamber when no free dispersion of rGO sheets was visible in the rGO solution, and were immediately immersed in water. A longer filtration time of about 8 hours was used for the Rw 1.72 rGO solution to completely remove water molecules in the system, yielding the Rw 1.72 rGO dry paper. It was reported that trapped water molecules could prevent the re-stacking of rGO sheets in the hydrogel papers, leading to a higher interlayer spacing.30 Three different types of hydrogel papers with varying reduction levels were obtained, i.e., Rw 1.72 rGO, Rw 0.52 rGO, and Rw 0.34 rGO hydrogel papers. Fig. 1 shows the SEM images of the cross-sectional views of hydrogel papers, which were freeze dried prior to being transferred into a vacuum chamber of SEM. The rGO sheets are well aligned in these papers. Some structural corrugations can be seen. Note that there is no visible structural difference for these three types of rGO papers, indicating that the different reduction levels of the rGO sheets have no significant effects on the microstructure of the obtained papers.To characterise the chemical functional groups in the rGO sheets, five different methods were employed, i.e., contact angle measurement, Fourier transform of infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and energy-dispersive X-ray spectroscopy (EDX).
Fig. 2 shows the contact angle of a 10 μl water droplet on the surfaces of as prepared rGO papers. The Rw 1.72 dry paper has a contact angle around 90°, indicating a hydrophobic nature of graphene, which is consistent with previous studies. For the Rw 1.72 hydrogel paper, the contact angle value is reduced to 68°. The excess water in the hydrogel papers should be the reason for this hydrophilic behaviour. For the Rw 0.52 and Rw 0.34 hydrogel papers, the contact angle reduces further to 54° and 51°, respectively. This is reasonable because it is well known that more oxygen functional groups in a graphene sheet can enhance its wettability.22
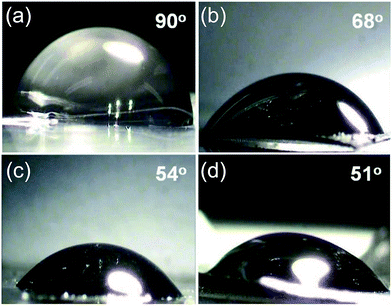 | ||
| Fig. 2 The contact angles of a 10 μl water droplet on (a) Rw 1.72 dry paper, (b) Rw 1.72, (c) Rw 0.52 and (d) Rw 0.34 hydrogel papers. | ||
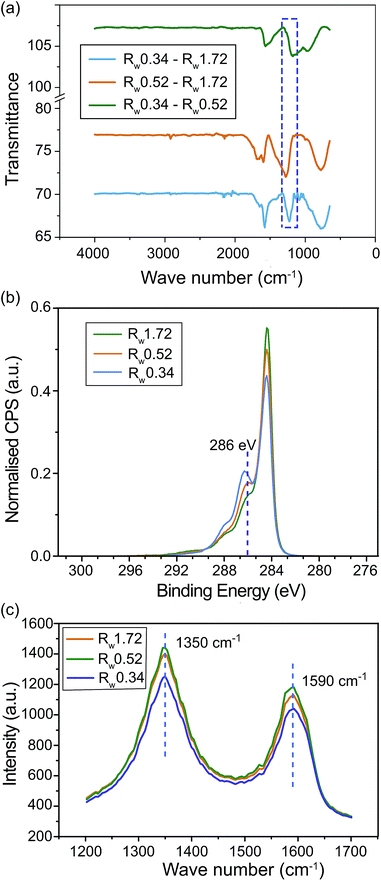
A comparison of FTIR spectra of the as-prepared hydrogel papers are depicted in Fig. 3(a). Differences in the FTIR spectra of the three types of hydrogel papers are presented in order to detect the differences in functional groups more clearly. The spectrum within the region from 1226 to 1277 cm−1 (highlighted using the dash box) corresponds to the vibrations of epoxy C–O bond.31–33 Compared with the Rw 1.72 case, the stronger spectrum intensities of the Rw 0.52 and Rw 0.34 hydrogel papers in this region indicate the existence of more C–O groups in the rGO sheets. This comparison also shows that the Rw 0.34 rGO hydrogel paper has more C–O groups than the Rw 0.52 hydrogel paper. This is as expected because more hydrazine reduction agent was used to produce the Rw 0.52 rGO sheets.
Table 1 summarises the quantitative information of atomic percentage of the rGO sheets, which were obtained from the XPS, EDX, and Raman spectroscopy analyses.
| Material | XPS | EDX | Raman | |||||
|---|---|---|---|---|---|---|---|---|
| C (%) | O (%) | N (%) | C–O/hydrocarbon ratio | C (%) | O (%) | N (%) | D/G ratio | |
| Rw 1.72 | 80.92 | 14.17 | 3.62 | 0.400 | 86.62 | 9.03 | 4.31 | 1.32 |
| Rw 0.52 | 79.02 | 16.54 | 3.78 | 0.531 | 75.79 | 16.06 | 8.14 | 1.29 |
| Rw 0.34 | 74.09 | 20.83 | 3.78 | 0.706 | 76.61 | 14.13 | 7.83 | 1.27 |
The elemental quantification obtained from XPS survey scans presented in Table 1 indicate a trend of decreasing O content with increasing weight ratio of hydrazine, as expected. High resolution C 1s spectral overlay of the three hydrogel paper samples are presented in Fig. 3(b). The overall peak shape is characteristic for partially reduced GO, with the main peak at 284.4 eV as expected for graphitic hydrocarbon and a shoulder at approximately 286 eV associated with C–O (and potentially C–N) groups. At higher binding energies we observe another less intense shoulder at approximately 288 eV associated with (N–)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–C–O and a tail in the spectra at binging energy >289 eV characteristic of rGO. It is apparent from the spectra overlay that the intensity of the shoulder associated with C–O decreases with increasing weight ratio of hydrazine, as expected and consistent with the elemental quantification results. In an attempt to quantify this trend, the high resolution C 1s spectra were fitted with a number of standard Gaussian–Lorentzian based components and the ratios of the components associated with C–O and hydrocarbon groups are presented in Table 1. As expected, sample Rw 0.34 has the largest ratio indicating it has the largest amount of C–O groups relative to hydrocarbon within the parameter space explored. Note that as only standard Gaussian–Lorentzian based components were used, the component for C–O groups is likely overestimated in all cases as the component related to sp2 carbon is lacking a tail that would extend to high binding energy, however the ratios still provide a useful method for comparing the hydrogel paper samples.
O and O–C–O and a tail in the spectra at binging energy >289 eV characteristic of rGO. It is apparent from the spectra overlay that the intensity of the shoulder associated with C–O decreases with increasing weight ratio of hydrazine, as expected and consistent with the elemental quantification results. In an attempt to quantify this trend, the high resolution C 1s spectra were fitted with a number of standard Gaussian–Lorentzian based components and the ratios of the components associated with C–O and hydrocarbon groups are presented in Table 1. As expected, sample Rw 0.34 has the largest ratio indicating it has the largest amount of C–O groups relative to hydrocarbon within the parameter space explored. Note that as only standard Gaussian–Lorentzian based components were used, the component for C–O groups is likely overestimated in all cases as the component related to sp2 carbon is lacking a tail that would extend to high binding energy, however the ratios still provide a useful method for comparing the hydrogel paper samples.
The Raman spectra for the three levels of reductions are presented in Fig. 3(c). These spectra display a clear D and G peaks at 1350 cm−1 and 1590 cm−1 respectively for all three samples. This is consistent with the standard spectrum seen for rGO.34 However, as reported in Table 1, slight variations of the D/G intensity ratios can be observed for the studied reduction levels. The slight perceptible decrease of D/G ratios indicate a decrease of sp2/sp3 bond ratios in the carbon structure due to the introduction of extra oxygen molecules to the rGO molecular structure.34–37 This indicates that the Rw 0.52 and Rw 0.34 has higher amount of carbon and oxygen interactions compared to that of Rw 1.72. This analysis further confirms that the Rw 0.34 samples have slightly more carbon oxygen interactions compared to Rw 0.52.
As summarised in Table 1, the semi-quantitative results obtained from EDX mapping shows a carbon, oxygen and nitrogen content of 86.7%, 9% and 4.3% respectively in Rw 1.72 hydrogel papers. This is consistent with the EDX results of graphene papers reported before.30 Although hydrazine hydrate can remove C–O–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–C
O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups efficiently during the reduction process, clear traces of C–OH groups are still found even at high reduction levels such as the case of Rw 1.72.25,32 The less reduction agent used in the reduction process left more functional groups in the rGO sheets as seen in Fig. 3(c), increasing the oxygen content in Rw 0.34 and Rw 0.52 to 14.13% and 16.06%, respectively. A slightly higher oxygen content in the Rw 0.52 paper (than that in the Rw 0.34 paper) appears contrary to the contact angle measurements as well as the FTIR, XPS, and Raman analysis results, and the fact that more reduction agent was used to produce the Rw 0.52 rGO sheets. We believe that such a discrepancy can be attributed to the limitations of the semi-quantitative EDX analysis. Nonetheless our EDX analysis yields two conclusions: (1) the Rw 0.52 and Rw 0.34 hydrogel papers have more functional groups than the Rw 1.72 papers; (2) there should be a minor difference of the content of oxygen functional groups in the Rw 0.52 and Rw 0.34 hydrogel papers.
O functional groups efficiently during the reduction process, clear traces of C–OH groups are still found even at high reduction levels such as the case of Rw 1.72.25,32 The less reduction agent used in the reduction process left more functional groups in the rGO sheets as seen in Fig. 3(c), increasing the oxygen content in Rw 0.34 and Rw 0.52 to 14.13% and 16.06%, respectively. A slightly higher oxygen content in the Rw 0.52 paper (than that in the Rw 0.34 paper) appears contrary to the contact angle measurements as well as the FTIR, XPS, and Raman analysis results, and the fact that more reduction agent was used to produce the Rw 0.52 rGO sheets. We believe that such a discrepancy can be attributed to the limitations of the semi-quantitative EDX analysis. Nonetheless our EDX analysis yields two conclusions: (1) the Rw 0.52 and Rw 0.34 hydrogel papers have more functional groups than the Rw 1.72 papers; (2) there should be a minor difference of the content of oxygen functional groups in the Rw 0.52 and Rw 0.34 hydrogel papers.
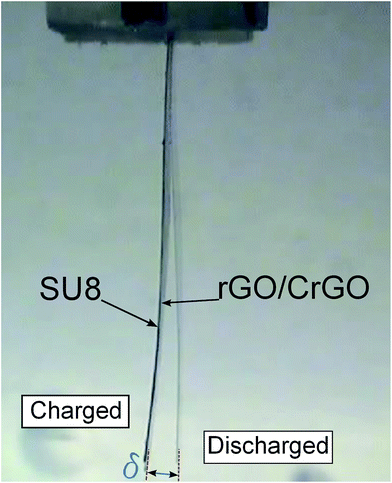
Next, to determine the electromechanical response of the graphene papers and to demonstrate their use as potential MEMS actuators, we fabricated a series of bimorph devices using SU8 as a polymer substrate as shown in Fig. 4. In the bimorph design, via tuning the thickness of rGO papers and substrates, the deflection can be enlarged to ensure an easy and accurate measurement. The surface dimensions of the device is 2 mm × 15 mm and the thickness is ∼90 μm for the dry paper devices and ∼220 μm for hydrogel paper devices. The bimorph device was mounted on a holder and immersed in 1 M NaCl aqueous solution. It was connected to an electrochemical station (Bio Logic VMP 300) as the working electrode using a platinum sheet and wires, meanwhile a platinum mesh and a saturated calomel electrode were used as the counter and reference electrodes, respectively. The chronoamperometry technique was employed to apply the square wave voltage starting from ±0.1 V up to ±1 V at increments of 0.1 V. Three charge/discharge cycles (with a period of 100 s) were conducted at a given voltage value. It should be noted that in this study the actuation characterisation was conducted at a low charging frequency of 0.01 Hz, providing sufficient time to achieve saturated actuation strain of the individual papers. Therefore, the electrical conductivity of rGO layers, which influences the actuation at high frequency, should play a minor role in our case. The deflection of the bimorph cantilevers were captured using a CCD camera (Watec; WAT-231S) mounted to a C-mount microscope (Infinity; InfiniVar CFM-2/S). The tip displacement of the bimorph cantilevers were measured using image processing techniques on the captured footage. For each type of rGO papers, the actuation experiments were repeated for 10 times using three to five bimorph devices.
The electromechanical actuation strain, ε of the active rGO papers can be calculated using the measured tip displacement of the bimorph cantilever, δ in the following equation,38
 | (1) |
In eqn (1), t1 and t2 are the thickness of the rGO paper layer and the SU8+epoxy layer, respectively, E1 and E2 are their Young's modulus, and L0 (15 mm) is the initial length of the cantilever. The value of t1 is 5 μm for the dry rGO papers and 150 μm for the hydrogel papers while their t2 values are ∼85 μm and ∼70 μm respectively. The Young's modulus of SU8 is 2 GPa (MICROCHEM, SU8 2000) and that of epoxy is 500 MPa (ref. 39) (Selleys, Aqua Repair). The E2 is calculated as the averaged Young's modulus of SU8 and epoxy layer based on their thickness: ∼1 GPa. Note that eqn (1) is widely used in micro/nano devices community, e.g., Al/SiO2, W/SiO2, Al/Si, Ni/Ni-diamond, SiO2/Ti bimorph beams.8,38,40
Fig. 5(a) shows the calculated actuation strain of the rGO papers as a function of an applied periodic square wave voltage at a frequency of 0.01 Hz (ESI†). A nearly quadratic strain–voltage relation was observed for all devices. The Rw 1.72 dry papers have an averaged maximum actuation strain of 0.037% at −1 V. The actuation strain is improved to ∼0.10% at −1 V for the Rw 1.72 rGO hydrogel papers. The 1.7 fold increase indicates that increasing interlayer distances between the rGO sheets in the papers is very important to improve actuation performance. Our Rw 1.72 hydrogel papers have a similar actuation strain to graphene papers from Liang et al.14 It should be emphasised that in our experiments no alien nanoparticles were used to keep the interlayer distances of our hydrogel papers.
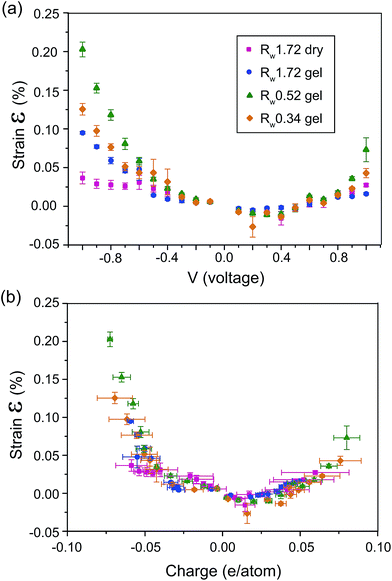 | ||
| Fig. 5 The electromechanical actuation strain vs. (a) applied voltage and (b) injected charges for the rGO papers. | ||
While following the similar quadratic behaviour against the applied potential, the Rw 0.52 and Rw 0.34 hydrogel papers unveil an averaged maximum strain performance of 0.20% and 0.13%, respectively. This is a profound enhancement in comparison with the highly reduced Rw 1.72 papers. The enhancement is about 100% for Rw 0.52 papers and about 40% for the Rw 0.34 papers compared to the actuation strain of Rw 1.72 hydrogel paper. This evidently proves that controlling the reduction level is indeed a very convenient and effective way to improve the electromechanical actuation performance of rGO papers.
Previous studies have shown that the amount of injected charges dominate the electromechanical strain arising from the EDL effect.18,41 Hence, we integrated the measured current with respect to time to estimate the charges stored in the papers. Fig. 5(b) illustrates the strain against the respective charge per atom. At a given voltage, the Rw 1.72 dry papers store much less charge than the hydrogel papers do. This can be attributed to the much less accessible surfaces in the dry papers. The more charge accumulation in hydrogel papers enhance the coulomb forces in the EDL, generating a large driving force for mechanical deformation. At −1 V, the Rw 0.52 and Rw 0.34 hydrogel papers store similar charges: −0.072 vs. −0.069 electrons per atom. In spite of a small ∼4.3% difference in the charge values, there is a considerable ∼54% difference in the actuation strain results. This evidence indicates that the amount of injected charges (per atom) is not the only factor that governs the electromechanical actuation of our rGO papers.
The ion intercalation, electric double layer effect and the quantum mechanical effect are reported as the mechanisms of the electromechanical actuation of carbon based materials.10,18,42 The graphene paper is composed of face-to-face aligned atomically thin graphene layers. Thus the ion intercalation between graphene layers would change the dimension perpendicular to the basal plane.42,43 The change in the basal plane arising from ion intercalation should be minor. Theoretical studies showed that the quantum mechanical effect should lead to extension upon electron doping and contraction upon hole doping and the overall strain–charge relation exhibited a highly non-parabola shape,10,18,43 which are clearly different from our experiments. On the contrary, theoretically the EDL effect endows a quadratic strain–charge relation.18,44 In addition, our previous ab initio simulations indicated that for graphene layers immersed in electrolyte, the EDL effect is much more significant than the quantum mechanical effect. The EDL effect, therefore, is highly likely to be the dominant mechanism in our system.
In an attempt to identify the key physical factors that determine the actuation arising from EDL effect, a theoretical model will be developed in the following. Following Riemenschneider et al.,41 the energy of the system can be expressed as,
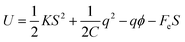 | (2) |
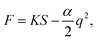 | (3) |
 | (4) |
Assuming that the change of capacitance C in comparison with the initial value C0 solely arises from the deformation S, we can make some simplifications for α. The electric capacitance can be estimated as 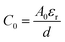 and
and 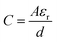 , in which A0 or A are the accessible surface area at the initial or final states, εr is the dielectric constant, and d is the thickness of the EDL. The surface area A can be expressed by using A0 and the electromechanical strain ε of the rGO papers as A0 = A(1 + 2ε). Thus the coefficient α is approximately α ≈ 2ε/C0S.
, in which A0 or A are the accessible surface area at the initial or final states, εr is the dielectric constant, and d is the thickness of the EDL. The surface area A can be expressed by using A0 and the electromechanical strain ε of the rGO papers as A0 = A(1 + 2ε). Thus the coefficient α is approximately α ≈ 2ε/C0S.
Given that at static equilibrium the internal forces (F) reduce to zero, from eqn (3) we obtain,
 | (5) |
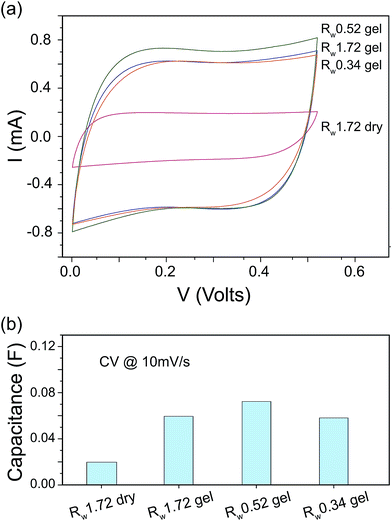
To examine the obtained relation eqn (5), we measured the capacitance and the stiffness of our rGO papers. Fig. 6(a) shows the current vs. voltage results of the rGO papers from the CV analysis. The calculated device capacitance are shown in Fig. 6(b). The hydrogel papers always have a higher electrochemical capacitance than the dry paper. This explains why the rGO dry paper bimorph device accumulated less charges at −1 V than those hydrogel papers (Fig. 5). Interestingly the Rw 0.52 rGO hydrogel paper has a slightly higher capacitance than both the Rw 1.72 and the Rw 0.34 hydrogel papers.
Fig. 7 depicts the obtained stress vs. strain relations in the tensile test and summarises the Young's modulus and stiffness results that were fitted using the tensile experimental data in Fig. 7(a) with strain values below 0.2%. The Rw 1.72 dry paper has the highest Young's modulus and stiffness, i.e., 2654 MPa and 1770 N m−1. Overall the hydrogel papers have a comparable Young's modulus and stiffness, in which the Rw 1.72 hydrogel paper has the highest values of modulus and stiffness and the Rw 0.52 rGO paper has the lowest ones. The low stiffness implies the lack of hindrance against the in-plane structural deformation, which should benefit the electro-chemical actuation strain output.
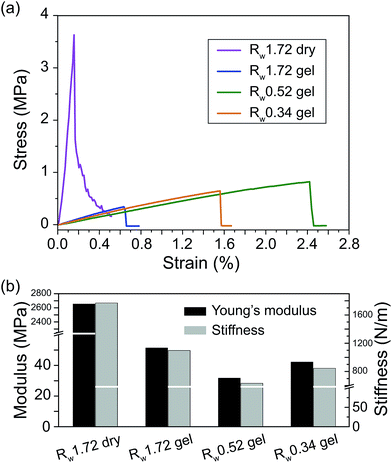 | ||
| Fig. 7 (a) The stress–strain relation of the rGO papers under a tensile loading. (b) The calculated Young's modulus and stiffness results of the rGO papers. | ||
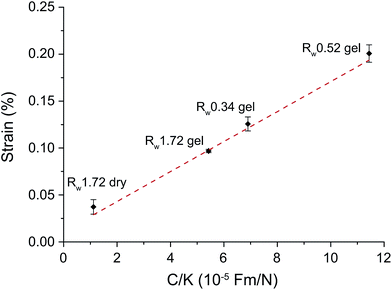
With the obtained capacitance C and stiffness K results, we plot the calculated electromechanical strain results at −1 V (Fig. 5) against the C/K values in Fig. 8. The results of the all four materials exhibit a very good linear relation, which is consistent with the derived relation eqn (5). Hence, we can conclude that indeed EDL is the dominant actuation mechanism for rGO papers and the capacitance and stiffness are two key parameters in determining the electromechanical actuation performance.
3 Conclusions
In summary, this study is devoted to enhance the electromechanical actuation performance of rGO papers via fine tuning the content of chemical functional groups in the rGO sheets. The effects of controlling the interlayer distance between rGO sheets were investigated as well. Through careful management of the amount of reduction agent in the chemical reduction process, three different types of rGO sheets were fabricated, i.e., Rw 1.72, Rw 0.52, and Rw 0.34 rGO. Three types of rGO hydrogel papers and one rGO dry paper were then produced using the vacuum filtration technique. The electromechanical actuation of some bimorph devices, which is composed of a layer of obtained rGO paper on top of a SU8+epoxy substrate, were then conducted to examine the actuation strains of the rGO papers. A quadratic strain-vs.-voltage relation and a quadratic strain-vs.-charge relation were obtained. We find that the Rw 0.52 and Rw 0.34 rGO hydrogel papers exhibit significantly enhanced actuation strains, suggesting fine tuning oxygen functional groups is a convenient and an effective way to achieve a better actuation performance. In-depth analysis reveals that two key parameters, electrochemical capacitance C and the stiffness K, govern the actuation strains arising from the EDL effect. A theoretical model is derived and its prediction agrees very well with our experimental results. The obtained in-depth understanding could lay a ground for future design and development of graphene based actuators.4 Experimental section
Material synthesis
Graphite oxide was synthesised from natural graphite (SP-1, Bay Carbon) by a modified Hummers method.45,46 As-synthesised graphite oxide was dispersed in water and subjected to dialysis to completely remove residual salts and acids. After that, the as-purified graphite oxide dispersion was then mixed in water to create a 0.5 wt% solution. The graphite oxide solution was then exfoliated to produce a GO solution by conducting ultrasonication for 30 min using a Branson Digital Sonifier (S450D, 500 W, 30% amplitude). The resultant GO solution was further diluted to achieve 0.288 mg ml−1 and subjected to 20 min of centrifugation at 4400 rpm to remove any unexfoliated graphite oxide using an Eppendorf 5702 centrifuge with a rotor radius of 14 cm. To achieve a maximum level of reduction, hydrazine with a weight ratio value of Rw = 1.72 (35 wt% in water, Sigma-Aldrich) was mixed to GO dispersion and the chemical reduction was conducted at 100 °C for 3 h. Controlled reduction of GO dispersion was conducted at 100 °C for 2 h by using the amount of hydrazine at Rw = 0.52 and Rw = 0.34. To avoid losing water molecules, the hydrogel papers were removed from the filtration chamber and immediately immersed in aqueous solution once no free dispersion of rGO sheets was visible in the rGO solution. A longer filtration time of about 8 hours was used for the Rw 1.72 rGO solution to completely remove water molecules in the system, yielding a Rw 1.72 rGO dry paper.Characterisation of rGO papers
FTIR was conducted using 64 scans on each sample. For each paper sample, five different regions in the cross-section were scanned to obtain the EDX mapping results. The standard cyclic voltammetry (CV) technique was conducted from 0 V to 0.5 V at a scan rate of 10 mV s−1 to determine the capacitance of the individual rGO paper (diameter = 5.83 mm) using a three electrode cell configuration. The mechanical tensile experiment was conducted using a dynamic mechanical analyzer (Rheometric Scientific, DMTA IV and software RSI Orchestrator) with a strain accuracy of 0.005% and a stress accuracy of 1 Pa at a strain ramp rate of 0.02% s−1. The rGO paper samples were cut with a blade into rectangular strips of approximately 4 mm × 10 mm. Raman spectroscopy was conducted with samples drop casted on to SiO2/Si substrates using WITec alpha 300R micro-confocal Raman system. The 532 nm laser was used to illuminate the sample surface through a 100× objective (NA = 0.9). During spectrum acquisition 600 line per mm grating was used at the same level of laser output, spectrum integration and accumulation times (60 second and 30 times per spectrum). XPS analysis was performed using an AXIS Nova spectrometer (Kratos Analytical Inc., Manchester, UK) with a monochromated Al KÎś source at a power of 180 W (15 kV × 12 mA) and a hemispherical analyser operating in the fixed analyser transmission mode. The total pressure in the main vacuum chamber during analysis was typically between 10−9 and 10−8 mbar. Survey spectra were acquired at a pass energy of 160 eV. To obtain more detailed information about chemical structure, oxidation states etc., high resolution spectra were recorded from individual peaks at 40 eV pass energy (yielding a typical peak width for polymers of 1.0 eV). Each specimen was analysed at an emission angle of 0° as measured from the surface normal. Assuming typical values for the electron attenuation length of relevant photoelectrons the XPS analysis depth (from which 95% of the detected signal originates) ranges between 5 and 10 nm for a flat surface. As the actual emission angle is ill-defined for rough surfaces (ranging from 0° to 90°), the sampling depth may range from 0 nm to approx. 10 nm. All elements present were identified from survey spectra and data processing was performed using CasaXPS processing software version 2.3.15 (Casa Software Ltd, Teignmouth, UK). The atomic concentrations of the detected elements were calculated using integral peak intensities and the sensitivity factors supplied by the manufacturer. Binding energies were referenced to the main C 1s peak (graphitic carbon) at 284.4 eV.Device fabrication
SU8 films of a thickness of 20 μm were spin coated on Si wafer covered with a Teflon layer. A set of SU8 cantilevers were then fabricated via photolithography techniques using our designed photo-masks. Next, a thin uniform epoxy layer was applied on top of the SU8 cantilevers using the Dr Blade technique, which enabled good adhesion of the rGO papers on top of it. The obtained bimorph devices have a surface dimension of 2 mm × 15 mm. These bimorph devices were transferred onto a glass slide support in the following electromechanical actuation experiments.Acknowledgements
The authors are grateful to Monash University Engineering faculty seed grants (2013/2014) for providing funding. The authors thank Dr Adrian Neild and Dr Mainak Majumder for their supports. This work was performed in part at the Monash Centre for Electron Microscopy (MCEM) and the Melbourne Centre for Nanofabrication (MCN) in the Victorian Node of the Australian National Fabrication Facility (ANFF). DL and JZL acknowledge the financial support from ARC Discovery projects.References
- P. Kim and C. M. Lieber, Science, 1999, 286, 2148 CrossRef CAS.
- A. Fennimore, T. Yuzvinsky, W.-Q. Han, M. Fuhrer, J. Cumings and A. Zettl, Nature, 2003, 424, 408 CrossRef CAS PubMed.
- S. V. Ahir and E. M. Terentjev, Nat. Mater., 2005, 4, 491 CrossRef CAS PubMed.
- J. E. Jang, S. N. Cha, Y. J. Choi, D. J. Kang, T. P. Butler, D. G. Hasko, J. E. Jung, J. M. Kim and G. A. Amaratunga, Nat. Nanotechnol., 2007, 3, 26 CrossRef PubMed.
- J. D. Madden, N. A. Vandesteeg, P. A. Anquetil, P. G. Madden, A. Takshi, R. Z. Pytel, S. R. Lafontaine, P. A. Wieringa and I. W. Hunter, IEEE J. Oceanic Eng., 2004, 29, 706 CrossRef.
- G. W. Rogers and J. Z. Liu, Appl. Phys. Lett., 2013, 102, 021903 CrossRef PubMed.
- T. Mirfakhrai, J. D. Madden and R. H. Baughman, Mater. Today, 2007, 10, 30 CrossRef CAS.
- Y. Huang, J. Liang and Y. Chen, J. Mater. Chem., 2012, 22, 3671 RSC.
- J. Liang, L. Huang, N. Li, Y. Huang, Y. Wu, S. Fang, J. Oh, M. Kozlov, Y. Ma and F. Li, et al., ACS Nano, 2012, 6, 4508 CrossRef CAS PubMed.
- R. H. Baughman, C. Cui, A. A. Zakhidov, Z. Iqbal, J. N. Barisci, G. M. Spinks, G. G. Wallace, A. Mazzoldi, D. De Rossi and A. G. Rinzler, et al., Science, 1999, 284, 1340 CrossRef CAS.
- M. Tahhan, V.-T. Truong, G. M. Spinks and G. G. Wallace, Smart Mater. Struct., 2003, 12, 626 CrossRef CAS.
- M. Hughes and G. M. Spinks, Adv. Mater., 2005, 17, 443 CrossRef CAS PubMed.
- Y. Yun, V. Shanov, Y. Tu, M. J. Schulz, S. Yarmolenko, S. Neralla, J. Sankar and S. Subramaniam, Nano Lett., 2006, 6, 689 CrossRef CAS PubMed.
- J. Liang, Y. Huang, J. Oh, M. Kozlov, D. Sui, S. Fang, R. H. Baughman, Y. Ma and Y. Chen, Adv. Funct. Mater., 2011, 21, 3778 CrossRef CAS PubMed.
- S.-E. Zhu, R. Shabani, J. Rho, Y. Kim, B. H. Hong, J.-H. Ahn and H. J. Cho, Nano Lett., 2011, 11, 977 CrossRef CAS PubMed.
- S. Park, J. An, J. W. Suk and R. S. Ruoff, Small, 2010, 6, 210 CrossRef CAS PubMed.
- J. Oh, M. E. Kozlov, J. Carretero-González, E. Castillo-Martínez and R. H. Baughman, Chem. Phys. Lett., 2011, 505, 31 CrossRef CAS PubMed.
- G. W. Rogers and J. Z. Liu, J. Am. Chem. Soc., 2011, 133, 10858 CrossRef CAS PubMed.
- Z. Chang, W. Yan, J. Shang and J. Z. Liu, Appl. Phys. Lett., 2014, 105, 023103 CrossRef PubMed.
- Y. Zhao, L. Song, Z. Zhang and L. Qu, Energy Environ. Sci., 2013, 6, 3520 CAS.
- X. Yang, L. Qiu, C. Cheng, Y. Wu, Z.-F. Ma and D. Li, Angew. Chem., Int. Ed., 2011, 50, 7325 CrossRef CAS PubMed.
- X. Xie, L. Qu, C. Zhou, Y. Li, J. Zhu, H. Bai, G. Shi and L. Dai, ACS Nano, 2010, 4, 6050 CrossRef CAS PubMed.
- X. Xie, H. Bai, G. Shi and L. Qu, J. Mater. Chem., 2011, 21, 2057 RSC.
- G. W. Rogers and J. Z. Liu, J. Am. Chem. Soc., 2012, 134, 1250 CrossRef CAS PubMed.
- G. He, H. Chen, J. Zhu, F. Bei, X. Sun and X. Wang, J. Mater. Chem., 2011, 21, 14631 RSC.
- X. Yu, H. Cai, W. Zhang, X. Li, N. Pan, Y. Luo, X. Wang and J. Hou, ACS Nano, 2011, 5, 952 CrossRef CAS PubMed.
- D. Li, M. B. Müller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101 CrossRef CAS PubMed.
- H. Chen, M. B. Müller, K. J. Gilmore, G. G. Wallace and D. Li, Adv. Mater., 2008, 20, 3557 CrossRef CAS PubMed.
- C. Cheng and D. Li, Adv. Mater., 2013, 25, 13 CrossRef CAS PubMed.
- X. Yang, C. Cheng, Y. Wang, L. Qiu and D. Li, Science, 2013, 341, 534 CrossRef CAS PubMed.
- S. Park, J. An, I. Jung, R. D. Piner, S. J. An, X. Li, A. Velamakanni and R. S. Ruoff, Nano Lett., 2009, 9, 1593 CrossRef CAS PubMed.
- P.-G. Ren, D.-X. Yan, X. Ji, T. Chen and Z.-M. Li, Nanotechnology, 2011, 22, 055705 CrossRef PubMed.
- V. Loryuenyong, K. Totepvimarn, P. Eimburanapravat, W. Boonchompoo and A. Buasri, Adv. Mater. Sci. Eng., 2013, 1 CrossRef PubMed.
- C. Mattevi, G. Eda, S. Agnoli, S. Miller, K. A. Mkhoyan, O. Celik, D. Mastrogiovanni, G. Granozzi, E. Garfunkel and M. Chhowalla, Adv. Funct. Mater., 2009, 19, 2577 CrossRef CAS PubMed.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 14095 CrossRef CAS.
- C. Gómez-Navarro, R. T. Weitz, A. M. Bittner, M. Scolari, A. Mews, M. Burghard and K. Kern, Nano Lett., 2007, 7, 3499–3503 CrossRef PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhang and R. Marcus, J. Microelectromech. Syst., 1999, 8, 43 CrossRef.
- R. E. Robertson and R. J. Buenker, J. Polym. Sci., Part A: Gen. Pap., 1964, 2, 4889 CrossRef CAS PubMed.
- S. Scott, J.-I. Kim, F. Sadeghi and D. Peroulis, J. Microelectromech. Syst., 2012, 21, 161–170 CrossRef CAS.
- J. Riemenschneider, S. Opitz, M. Sinapius and H. P. Monner, J. Intell. Mater. Syst. Struct., 2009, 20, 245 CrossRef CAS PubMed.
- L. Lu, J. Liu, Y. Hu and W. Chen, Chem. Commun., 2012, 48, 3978–3980 RSC.
- G. Sun, J. KÃijrti, M. Kertesz and R. H. Baughman, J. Am. Chem. Soc., 2002, 124, 15076–15080 CrossRef CAS PubMed.
- L. Pastewka, P. Koskinen, C. Elsässer and M. Moseler, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 155428 CrossRef.
- N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Chem. Mater., 1999, 11, 771 CrossRef CAS.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09743f |
| This journal is © The Royal Society of Chemistry 2015 |