Bio-inspired laminated graphite nanosheets/copper composites
Abstract
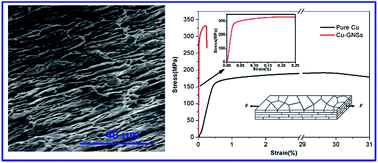
As one of the most industrially important metals, copper (Cu) was successfully reinforced with graphite nanosheets (GNSs). A nacre-inspired laminated GNSs/Cu composite material was fabricated by a combination of ball-milling and hot-rolling techniques. During the ball-milling process, the GNSs were in situ produced from graphite exfoliation. The Cu–GNSs composite ingot was hot-rolled into a belt to form a laminated structure. The laminated Cu–GNSs composite material showed improved mechanical properties observed from tensile and three-point bending tests. The Young's modulus of Cu–GNSs composites was up to 170 GPa and the bending strength reached 660 MPa. This processing route was also advantageous in low-cost, mass-producing manufacture.

 Please wait while we load your content...
Please wait while we load your content...