Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rational design of a fluopyram hapten and preparation of bioconjugates and antibodies for immunoanalysis†
E. Ceballos-Alcantarillaa,
C. Agullóa,
A. Abad-Fuentesb,
A. Abad-Somovilla*a and
J. V. Mercader*b
aDepartment of Organic Chemistry, Universitat de València, Doctor Moliner 50, Burjassot, 46100, València, Spain. E-mail: antonio.abad@uv.es
bDepartment of Biotechnology, Institute of Agrochemistry and Food Technology, Consejo Superior de Investigaciones Científicas (IATA–CSIC), Agustí Escardino 7, Paterna, 46980, València, Spain. E-mail: jvmercader@iata.csic.es
First published on 3rd June 2015
Abstract
A fluopyram hapten was designed in which insignificant electronic and structural modifications were foreseen and all potentially interacting chemical moieties were maintained. This hapten was prepared by total synthesis and three immunologically active bioconjugates were obtained and characterized. High-affinity and specific antibodies to fluopyram were raised.
Small organic chemicals constitute particular immunochemical targets because they are haptens from the immunological point of view. This means that a mimic of the analyte needs to be covalently coupled to a carrier macromolecule in order to generate antibodies by animal immunization. Most commonly, functionalized derivatives of the target compound holding a spacer arm are prepared in the laboratory because no reactive chemical groups are available in the molecule, or just because it is not advisable to modify potential antigenic determinants. The resulting synthetic hapten should preserve as much as possible the electronic and conformational properties of the target compound. Linear saturated aliphatic bridges with 3 to 6 carbon atoms are generally preferred for minimum physicochemical and antigenic interferences over the hapten or the immune system, respectively. Unfortunately, the optimum linker tethering site for immunizing bioconjugate preparation is difficult to predict.1 Substitution of a C–H bond by a C–C bond in hapten synthesis has afforded excellent immunochemical binders,2 though other approaches have also been successful.3 From our experience, the spacer should be better placed at the edge of molecular longitudinal axis, at distal positions from characteristic chemical moieties. On the contrary, positions next to highly antigenic groups or central locations with respect to the molecular longitudinal axis seem to be detrimental for antibody affinity and specificity.4 Furthermore, introduction at the right position of a hydrocarbon arm holding a reactive group is not so straightforward for many compounds, thus total synthesis is usually compulsory in such cases. For coupling to carrier proteins, carboxyl is the preferred moiety because it is a relatively robust group, compatible with a variety of reaction conditions, and it is readily activated to react in aqueous media with primary amine groups which are usually abundant in proteins as lysine side chains. However, severe molecular charge changes could destabilize the resulting bioconjugate, so moderate hapten densities are recommended.
Succinate dehydrogenase, also called respiratory complex II, is a fundamental constituent of the mitochondrial electron transport chain and the tricarboxylic acid cycle of fungi. Since the late 1960s, succinate dehydrogenase inhibitors (SDHI) have been employed to fight fungal diseases.5 In recent years, new-generation SDHI fungicides with extended biocide properties have been developed. Novel SDHIs include fluopyram, penthiopyrad, fluxapyroxad, and boscalid which are characterized by a higher efficiency and a broader spectrum of anti-mycotic activity. Fluopyram was first commercially registered in the United States in 2012![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and approved in the European Union in 2014.7 Structurally, it is characterized by two trifluoromethyl substituents and a diaryl aromatic system connected by a propanamide bridge (Fig. 1). Since no active chemical groups are available for conjugation to carrier macromolecules, introduction of a functional spacer arm is compulsory in order to prepare immunogenic and antigenic fluopyram bioconjugates.
6 and approved in the European Union in 2014.7 Structurally, it is characterized by two trifluoromethyl substituents and a diaryl aromatic system connected by a propanamide bridge (Fig. 1). Since no active chemical groups are available for conjugation to carrier macromolecules, introduction of a functional spacer arm is compulsory in order to prepare immunogenic and antigenic fluopyram bioconjugates.
The aim of the present study was to evaluate the capacity of a fluopyram derivative, obtained by total synthesis, to elicit high-affinity and specific antibodies. Conformational studies together with electronic analysis were carried out for hapten suitability assessment. Immunochemically active biomolecules were prepared using the purified active ester of the hapten in order to better modulate the final hapten-to-protein molar ratio. The immune response to a synthetic bioconjugate was assessed from rabbit antisera collected at different stages of immunization. Polyclonal antibodies were partially purified and the affinity and specificity to fluopyram was estimated by competitive enzyme-linked immunosorbent assay (cELISA), using the antibody-coated direct and the conjugate-coated indirect formats.
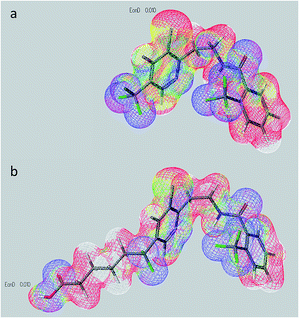
Molecular modelling techniques have been explored by several research groups for hapten design.8 In order to introduce minimum electronic and structural modifications to the fluopyram framework, a novel approach was envisaged, i.e., substitution of a C–F bond by a C–C bond. An analogous molecule was designed (hapten FPa, Fig. 1), wherein the hydrocarbon chain constituting the spacer arm was incorporated via formal replacement of a trifluoromethyl fluorine atom. Such modification of the trifluoromethyl moiety had a limited effect on the electronic distribution on the fluopyram molecule – only a significant alteration on the directly involved carbon atom (C1) was observed (Fig. 1). Moreover, the linker was located at the edge of the molecular longitudinal axis, such that, presumably, it did not hamper the fluopyram framework in the hapten to cover the same conformational space as the fluopyram molecule itself (Fig. 2). In principle, other aspects that are fundamental for the generation of the antibody binding pocket, which are hydrophobic interactions and low-energy interactions such as hydrogen bonding, are minimally affected by this structural modification, since the remaining difluoromethyl group (CF2) shows a high lipophilicity,9 similar to the CF3 group, and it is also able to act as a weak hydrogen acceptor through hydrogen bonding.10
The synthesis of hapten FPa is outlined in Scheme 1. It was based on the initial preparation of a dichloropyridine bearing a lineal keto-carboxylate chain at the C-3 position of the pyridine ring (compound 4). Its preparation was carried out by a Pd-catalyzed cross-coupling reaction11 between nicotinoyl chloride 2 and the functionalized alkylzinc reagent 3, which was readily prepared immediately before use by reaction of methyl 5-iodopentanoate12 and activated zinc dust in the presence of 1,3-dimethyl-2-imidazolinone. Next, the keto-carbonyl group of 4 was transformed into the corresponding gem-difluoro group using the electrophilic fluorinating agent diethylaminosulfur trifluoride, thus completing the hapten linking arm. Microwave-promoted regioselective aromatic nucleophilic substitution at the C-6 position of dichloropyridine 5 with tert-butyl cyanoacetate in the presence of cesium carbonate, followed by decarboxylation under acidic conditions, afforded the substituted pyridin-acetonitrile 7 that was transformed into the amino hydrochloride 8 via catalytic hydrogenation of the nitrile group in methanolic HCl. The entire carbon framework of the target hapten was completed by a reaction of amidation between the amino group of 8 and the benzoic acid 9 mediated by the phosphonium salt coupling reagent PyAOP.13 The synthesis of hapten FPa was finished by hydrolysis of the methyl ester moiety of 10 to the corresponding acid under standard basic conditions. Overall, the synthesis of hapten FPa proceeded in 8 steps with a total yield of ca. 10%. Full experimental synthetic details and spectroscopic data can be found in the ESI.†
Prior to protein coupling, the synthetic hapten was straightforward activated and readily purified. Active ester formation was carried out with N,N-disuccinimidyl carbonate during 4 h at room temperature. This scarcely used approach in immunochemistry14 affords high synthetic yields and the corresponding ester can be easily purified due to the absence of relevant by-products. An adapted procedure of previous studies was followed.15 The reaction mixture was cleaned-up by liquid–liquid extraction affording the N-hydroxysuccinimide ester of hapten FPa in nearly quantitative yield. Activation protocol and spectra of the corresponding succinimidyl active ester can be found in the ESI.† As a consequence of using pure activated hapten, high-yield coupling reactions to proteins with minute amounts of hapten could be performed at room temperature in just 2 h. Moreover, no secondary undesirable reactions occurred, thus the same hapten activation strategy could be applied for preparing all bioconjugates. As carrier proteins, bovine serum albumin (BSA), ovalbumin (OVA), and horseradish peroxidase (HRP) for immunogen, coating antigen, and enzyme tracer preparation were respectively employed. The resulting bioconjugates were purified by size exclusion chromatography. Highly suitable hapten-to-protein molar ratios of ca. 16, 4, and 1 for BSA–FPa, OVA–FPa, and HRP–FPa conjugates, respectively, were obtained, as determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Complete coupling procedures and MALDI mass spectrometry analysis (Fig. S1†) of the prepared biomolecules are included in the ESI.†
Two rabbits were immunized with 300 μg of BSA bioconjugate and antisera were obtained following established procedures16 in compliance with the laws and guidelines of the Spanish Ministry of Agriculture, Food, and Environment, and approved by the Ethics Committee of the University of Valencia (permit number: A1329731961154). Blood samples were taken from the ear vein after the third injection, and total sera were collected 10 days after the final boost. Immunoglobulins were partially purified by double salting out with saturated ammonium sulphate. Immunization was confirmed and antibodies were characterized by checkerboard antibody-coated direct and antigen-coated indirect cELISA (see immunoassay protocols in the ESI†). Standards, including a blank, were prepared in borosilicate glass vials by serially diluting fluopyram in 10 mM phosphate buffer, pH 7.4, containing 140 mM NaCl, starting from a 10 μM fluopyram solution in the same buffer. Coated microplates at 3 × 103-, 1 × 104-, or 3 × 104-fold antibody dilution and 100 or 1000 ng mL−1 bioconjugate concentration were prepared. A standard curve was run in each plate column, and increasing tracer concentrations (from 3 to 300 ng mL−1) or antibody dilutions (from 3 × 103-fold to 3 × 105-fold) were evaluated for direct and indirect cELISA, respectively, in different plate columns. Therefore, a series of inhibition curves with varying maximum absorbance values at zero dose of analyte (Amax) were obtained for each antibody in every assay format. Mean absorbance values were plotted against the logarithm of fluopyram concentration and experimental data were fitted to a four-parameter logistic equation using the SigmaPlot software, version 12.5 (Systat Software Inc., Chicago, IL, USA). The concentration of fluopyram affording a 50% inhibition of the maximum signal (IC50) was taken as an estimation of antibody affinity.
The immunogenic capacity of the prepared immunoreagents was assessed by checkerboard cELISA employing bioconjugates OVA–FPa and HRP–FPa as counterpart antigen, depending on the assay format as described above. Antisera were sampled after the third injection, and titres and affinities were determined. At this stage, regular antisera titres (1/104 or 1/3 × 104) were found in order to achieve Amax values between 0.8 and 1.5 at zero dose of analyte, and IC50 values were acceptable, or even better than usual for antiserum #2 with the direct cELISA (Table 1). Besides, the immune response was evaluated after the fourth injection. As expected, titres were similar to those of third-injection blood samples but the affinity of both antisera had significantly increased. Now, the generated antisera showed IC50 values in the low nanomolar range, particularly with the direct immunoassay format. Antibody FPa#2 showed the highest affinity to fluopyram (IC50 = 3.4 nM). The inhibition curve for fluopyram using antibody FPa#2 and tracer HRP–FPa obtained by direct cELISA can be seen in Fig. S2.† Moreover, specificity of both antisera was studied and no cross-reactivity was observed with other fungicides of the same family (penthiopyrad and fluxapyroxad), fungicides with structural similarities (fluopicolide and trifloxystrobin), and the main metabolite, i.e. 2-(trifluoromethyl)-benzamide, commonly referred to as fluopyram-benzamide or M25.17
| Rabbit | Third injection | Fourth injection | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct | Indirect | Direct | Indirect | |||||||||
| [As]a | [HRP]b | IC50c | [As] | [OVA] | IC50 | [As] | [HRP] | IC50 | [As] | [OVA] | IC50 | |
| a Antiserum dilution × 103.b Assay bioconjugate concentration in ng mL−1.c Values are in nM. | ||||||||||||
| #1 | 10 | 300 | 38.8 | 30 | 100 | 103.0 | 10 | 100 | 6.5 | 30 | 100 | 26.0 |
| #2 | 10 | 100 | 6.1 | 10 | 100 | 22.7 | 10 | 30 | 3.4 | 30 | 100 | 12.0 |
Conclusions
As far as we know, these are the first ever reported bioconjugates and antibodies for the new-generation fungicide fluopyram. Rational hapten design and total synthesis was carried out in order to obtain a suitable fluopyram derivative. A single, straightforward, and high-yield coupling strategy using pure active ester was applied for immunogenic and antigenic biomolecule preparation. Our results confirm the adequacy of the hapten functionalization strategy by substitution of a C–F bond by a C–C bond, and the competence of the produced bioconjugates for high-affinity and specific antibody generation – as predicted by computational evaluation and MALDI-TOF-MS analysis – as well as for antigenic application. As preliminary results, the immunosensing capability of the obtained antibodies has been studied.Acknowledgements
This work was supported by the Spanish Ministerio de Ciencia e Innovación (AGL2012-39965-C02-01) and cofinanced by European Regional Development Funds. E.C.-A. was a recipient of a predoctoral fellowship from the “Atracció de Talent, VLC-CAMPUS” program of the University of Valencia. Proteomic analysis was carried out in the SCSIE University of Valencia Proteomics Unit, a member of ISCIII ProteoRedProteomics Platform. We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).Notes and references
- A. Y. Moreno, A. V. Mayorov and K. D. Janda, J. Am. Chem. Soc., 2011, 133, 6587 CrossRef CAS PubMed; Z.-L. Xu, Y.-D. Shen, Y.-M. Sun, K. Campbell, Y.-X. Tian, S.-W. Zhang, H.-T. Lei and Y.-M. Jiang, Talanta, 2013, 103, 306 CrossRef PubMed.
- J. V. Mercader, F. A. Esteve-Turrillas, C. Agulló, A. Abad-Somovilla and A. Abad-Fuentes, Analyst, 2012, 137, 5672 RSC; E. Ceballos-Alcantarilla, A. Abad-Fuentes, V. Aloisio, C. Agulló, A. Abad-Somovilla and J. V. Mercader, Analyst, 2014, 139, 5358 RSC.
- J. V. Mercader and A. Abad-Fuentes, J. Agric. Food Chem., 2009, 57, 5129 CrossRef CAS PubMed; C. Suárez-Pantaleón, J. V. Mercader, C. Agulló, A. Abad-Somovilla and A. Abad-Fuentes, Org. Biomol. Chem., 2011, 9, 4863 Search PubMed; A. Abad-Fuentes, C. Agulló, F. A. Esteve-Turrillas, A. Abad-Somovilla and J. V. Mercader, J. Agric. Food Chem., 2014, 62, 2742 CrossRef PubMed.
- J. V. Mercader, C. Agulló, A. Abad-Somovilla and A. Abad-Fuentes, Org. Biomol. Chem., 2011, 9, 1443 Search PubMed; J. Parra, J. V. Mercader, C. Agulló, A. Abad-Fuentes and A. Abad-Somovilla, Tetrahedron, 2011, 67, 624 CrossRef CAS PubMed; F. A. Esteve-Turrillas, C. Agulló, A. Abad-Fuentes, A. Abad-Somovilla and J. V. Mercader, J. Agric. Food Chem., 2012, 60, 4803 CrossRef PubMed.
- H. F. Avenot and T. J. Michailides, Crop Prot., 2010, 29, 643 CrossRef CAS PubMed.
- T. J. Proffer, E. Lizotte, N. L. Rothwell and G. W. Sundin, Pest Manage. Sci., 2013, 69, 747 CrossRef CAS PubMed.
- EU Pesticide Database at: http://ec.europa.eu/sanco_pesticides/public/index.cfm?event=homepage%26language=EN(accessed April 2, 2015).
- C. A. Spinks, G. M. Wyatt, H. A. Lee and M. R. A. Morgan, Bioconjugate Chem., 1999, 10, 583 CrossRef CAS PubMed; E. C. Peterson, M. Gunnell, Y. Che, R. L. Goforth, F. I. Carroll, R. Henry, H. Liu and S. M. Owens, J. Pharmacol. Exp. Ther., 2007, 322, 30 CrossRef PubMed; D. G. Pinacho, F. Sánchez-Baeza and M.-P. Marco, Anal. Chem., 2012, 84, 4527 CrossRef PubMed.
- V. Prakash Redd, in Organofluorine Compounds in Biology and Medicine, Elsevier, Oxford, 2015, ch. 1, pp. 1–28 Search PubMed.
- C. Dalvit, C. Invernizzi and A. Vulpetti, Chem.–Eur. J., 2014, 20, 11058 CrossRef CAS PubMed.
- T. Iwai, T. Nakai, M. Mihara, T. Ito, T. Mizuno and T. Ohno, Synlett, 2009, 1091 CrossRef CAS PubMed.
- M. P. Glenn, M. J. Kelso, J. D. A. Tyndall and D. P. Fairlie, J. Am. Chem. Soc., 2003, 125, 640 CrossRef CAS PubMed.
- S.-Y. Han and Y.-A. Kim, Tetrahedron, 2004, 60, 2447 CrossRef CAS PubMed.
- G. Vaidyanathan and M. R. Zalutsky, Nat. Protoc., 2006, 1, 1655 CrossRef CAS PubMed; S. A. Hilderbrand, K. A. Kelly, M. Niedre and R. Weissleder, Bioconjugate Chem., 2008, 19, 1635 CrossRef PubMed; A. V. Ustinov, V. V. Shmanai, K. Patel, I. A. Stepanova, A. A. Prokhorenko, I. V. Astakhova, A. D. Malakhov, M. V. Skorobogatyi, P. L. Bernad Jr, S. Khan, M. Shahgholi, E. M. Southern, V. A. Korshun and M. S. Shchepinov, Org. Biomol. Chem., 2008, 6, 4593 Search PubMed.
- F. A. Esteve-Turrillas, J. Parra, A. Abad-Fuentes, C. Agulló, A. Abad-Somovilla and J. V. Mercader, Anal. Chim. Acta, 2010, 682, 93 CrossRef CAS PubMed; R. López-Moreno, J. V. Mercader, C. Agulló, A. Abad-Somovilla and A. Abad-Fuentes, Org. Biomol. Chem., 2013, 11, 7361 Search PubMed.
- F. A. Esteve-Turrillas, J. V. Mercader, C. Agulló, J. Marzo, A. Abad-Somovilla and A. Abad-Fuentes, Analyst, 2014, 139, 3636 RSC.
- European Food Safety Authority, EFSA J., 2014, 12(12), 3947. available online, www.efsa.europa.eu/efsajournal, (accessed April 2015) Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and instruments; hapten synthesis, activation, and coupling protocols; spectroscopic data; MALDI spectra of bioconjugates; antibody generation and competitive ELISA procedures; and copies of the 1H NMR spectra. See DOI: 10.1039/c5ra09124a |
| This journal is © The Royal Society of Chemistry 2015 |