Self-assembly of monolayered lipid membranes for surface-coating of a nanoconfined Bombyx mori silk fibroin film†
Abstract
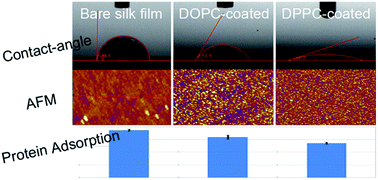
Regenerated Bombyx mori (B. mori) silk fibroin is a type of widely used biomaterial. The β-sheet structure of it after methanol treatment provides water-insolubility and mechanical stability while on the other side leads to a hydrophobic surface which is less preferred by biological systems. In this work we prepare a novel type of nanoconfined silk fibroin film with a thickness below 100 nm. The film has a flat while hydrophobic surface because of its β-sheet structure due to the z-direction confinement during formation. Different types of lipid monolayers, DOPC, DPPC and MO, are assembled on the silk film surface. The lipid coating, especially the DPPC membrane, provides a much smoother and more hydrophilic surface due to the gel phase tails of the lipids, in comparison with the DOPC and MO ones which are in a liquid phase and have a much stronger interfacial association between silk film surface and lipid tails. Such a lipid coating preserves the biocompatibility and cellular affinity of the silk film which promises potential applications as surface coatings for materials for biological use.

 Please wait while we load your content...
Please wait while we load your content...