Enzymatic delignification: an attempt for lignin degradation from lignocellulosic feedstock
Abstract
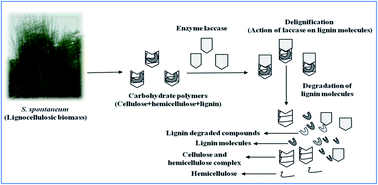
Burgeoning population growth and an increased demand for transportation and industrialization has led to the excessive use of fossil fuels, which in turn leads to higher levels of greenhouse gas emissions and contributes to global warming. At this juncture, biomass-based biofuel production from sustainable resources such as lignocellulosics acts as a better alternative for achieving zero emissions. This in turn necessitates the importance of development of an efficient biomass delignification method, which is an essential prerequisite for the complete biofuel production process. Lignocellulosics such as Saccharum spontaneum contain 17.46% of lignins and 67% of carbohydrates within its cell walls. To make this enormous amount of carbohydrates more accessible for hydrolysis and for further use in fermentation, lignin degradation through laccase has been carried out. In the present study, response surface methodology (RSM) based on central composite design (CCD) has been used to investigate the effects of the various process parameters. The maximum delignification obtained was 84.67% at 6.21 h of incubation time upon monitoring the initial lignin content of 17.46% of the biomass. Thorough study of the biomass was carried out by elemental composition analysis and energy density measurement. Further structural characteristics of the delignified substrate were analyzed by scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy and X-ray diffraction (XRD) spectroscopy, which supported the efficacy of the delignification process.

 Please wait while we load your content...
Please wait while we load your content...