Chain length effect on drug delivery of chrysin modified mPEG–PCL micelles
Yan Lianga,
Xinyu Penga,
Ying Chena,
Xin Denga,
Wenxia Gaoab,
Jun Caoa,
Jing Changc,
Zhongwei Gua and
Bin He*a
aNational Engineering Research Center for Biomaterials, Sichuan University, Chengdu 610064, China. E-mail: bhe@scu.edu.cn; Fax: +86-28-85412923; Tel: +86-28-85412923
bCollege of Chemistry & Materials Engineering, Wenzhou University, Wezhou 325035, China. E-mail: wenxiag@wzu.edu.cn; Fax: +86-532-82032105; Tel: +86-577-88368280
cCollege of Marine Life Science, Ocean University of China, Qingdao 266003, China. E-mail: jingjing_ch@yahoo.com.cn; Fax: +86-532-82032105; Tel: +86-532-82032109
First published on 1st July 2015
Abstract
Four chrysin modified mPEG–PCL block copolymers with different chain lengths of mPEG and PCL blocks were synthesized and self-assembled into micelles to load the anticancer drug doxorubicin (DOX). The effect of block chain length on drug delivery was investigated. The four block copolymers were characterized by 1H NMR, GPC and DSC. The drug loading contents of all the micelles were higher than 20%, the mPEG2k–PCL5k–CHS micelles showed the highest drug loading content and encapsulation efficiency of 26.8% and 93%, respectively. The micelles were spherical with the size increasing after drug encapsulation, and the mean size of the drug loaded micelles was around 100 nanometers. π–π stacking interactions between the micelles and DOX was invoked. The mPEG2k–PCL5k–CHS micelles exhibited the best profile for sustained-release. The cellular uptake and IC50 revealed that the DOX loaded mPEG2k–PCL5k–CHS micelles showed the best anticancer activity in vitro.
Introduction
Polymeric micelles have been attracted great interest from biomaterials scientists and pharmacists as nanocarriers for anticancer drug delivery due to their advantages of long circulation and easy functionalization.1 The unique core–shell architecture of polymeric micelles traps lipophilic anticancer drugs in the hydrophobic cores to enhance the solubility of anticancer drugs and protect the drugs to avoid degradation via enzymes in the delivery process in vivo.2 Clinical trials3 of polymeric micelles based nanomedicine has encouraged more and more researchers and pharmaceutical companies to focus on the development of polymeric micelle formulations.Amphiphilic poly(ethylene glycol)–poly(ε-caprolactone) (PEG–PCL) diblock copolymers could self-assembly into polymeric micelles in aqueous solution. As biodegradable and biocompatible polymers, PEG–PCL were extensively investigated for anticancer drug delivery.4 Exciting progresses were received in PEG–PCL polymeric micelle drug delivery systems for the specificity of hydrophobic PCL segments as cores, which were favorable for drug loading as well as release diffusion due to the coexistence of flexible amorphous and rigid crystal domains with low glass transition temperature (about −60 °C) and crystal melting temperature (about 60 °C).5 All kinds of anticancer drugs including doxorubicin,6 paclitaxel,7 camptothecin1 and 5-fluorouracil8 have been reported to load in PEG–PCL micelles for the treatment of different types of cancers on animal models.
To polymeric micelles, the balance between hydrophilic and hydrophobic segments decided the self-assembly behaviors and properties of micelles.9 With the adjustment of compositions and hydrophilic/hydrophobic balance of amphiphiles, the morphology of polymeric micelles could be controlled in spherical,10 rod,11 flow-like12 and worm.13 The balance between hydrophilic and hydrophobic segments also affected drug loading, release profile and anticancer activity.14 The chain length of PEG in PEG–PCL micelles was reported to act an important role in the fabrication of drug delivery systems.15
Amphiphilic copolymers aggregated together via self-assembly to form polymeric micelles,16 the weak physical interactions within polymeric micelles were considered not strong enough to maintain the aggregation, the dissociation was possibly occurred during the delivery in blood stream, thus, crosslink of shells or cores was carried out to stabilize the polymeric micelles,3 however, the solidified micelles were partially non-degradable and the hydrodynamic characteristics of polymeric micelles were seriously affected.17,18 In our previous work, we developed a new strategy to stabilize polymeric micelles with introducing π–π stacking interaction between hydrophobic moieties and anticancer drugs. A series of polymeric micelles were fabricated and the promising stabilization was exhibited.14,19–24 Recently, small molecules with different π-conjugated moieties of cinnamic acid, coumarin derivative and chrysin were immobilized on the terminal groups of PCL segments in PEG–PCL micelles to evoke π–π stacking interaction with anticancer drug doxorubicin, significant drug loading properties and anticancer activities both in vitro and in vivo were exhibited in chrysin modified PEG–PCL micelles.
In this paper, the chain length effects of chrysin modified PEG–PCL micelles on the drug delivery of doxorubicin were investigated in details. Four PEG–PCL diblocks copolymers with the block molecular weights of 2000 and 5000 were synthesized. The influence of both hydrophilic and hydrophobic chain length on the movement of copolymer chains, self-assembly, drug release profiles and in vitro anticancer activity of polymeric micelles were investigated.
Materials and method
Materials
α-Methoxy-poly(ethylene glycol)s (mPEG, Mw = 2000 and 5000 g mol−1), stannous octoate (Sn(Oct)2), ε-caprolactone (CL) and Hoechst were purchased from Sigma-Aldrich Co. Chrysin (CHS), methylbenzenesulfonyl (TsCl) and triethylamine (TEA) were purchased from Asta Tech Pharmaceutical (Chengdu, China) and used as received. Doxorubicin hydrochloride (DOX·HCl, Zhejiang Hisun Pharmaceutical China) was deprotonated according to the method previously reported.25 Dulbecco's Modified Eagle's Medium (DMEM), 100× mycillin, fetal bovine serum (FBS) and cell counting kit-8 (CCK-8) were purchased from HyClone Inc. and used for cytotoxicity test. All the solvents were purchased from Chengdu Kelong Chemical Co. (China) and purified before used.Characterizations
The 1H NMR spectra were performed on Bruker Avance II NMR spectrometer at 600 MHz using CDCl3 as solvents with 0.5% tetramethylsilane as the internal standard. Gel permeation chromatography (GPC) was employed to characterize the molecular weight and the molecular weight distribution. GPC analysis was performed on a SDV Linear M5μ8Χ300 mm column (Polymer Standard Service, Mainz, Germany) with chloroform as the eluent (1 mL min−1) and polystyrene standards for column calibration. The eluent was analyzed with a Waters HPLC system equipped with a model 1515 pump, a 717 plus autosampler, and a 2414 refractive index (RI) detector. The thermal properties of block copolymers were determined by differential scanning calorimetry (DSC, Q2000 TA Instruments), about 5 mg samples in hermetically closed aluminum pans were subjected to a heat–cool–heat program from −80 to +120 °C at a heating rate of 10 °C min−1 and a cooling rate of 5 °C min−1. The X-ray diffraction was recorded on X'Pert ProMPD. Dynamic light scattering (DLS) measurements were carried out using a dynamic light scattering spectrometer (Malvern ZetasizerNano ZS) to determine the size and size distribution of micelles. Transmission electron microscopy (TEM, FEI Tecnai G2 F20 S-TWIN) was used to observe the morphology of micelles. The TEM samples were prepared by dropping the fresh solution onto Quantifoil holey carbon foil (Micro Tools GmbH, Germany) and dried overnight at room temperature. The π–π interactions between DOX and micelles were determined by UV-vis absorption (Specord 200 PLUS) and fluorescence spectra (HITACHI F-700).Synthesis of mPEG–PCL diblock copolymers
mPEG–PCL diblock copolymers were synthesized by ring-opening polymerization of ε-CL with mPEG homopolymer as macroinitiator and Sn(Oct)2 as the catalyst. Prescribed amount of mPEG, ε-CL and Sn(Oct)2 (ca. 0.1% of ε-CL in molar amount) were mixed in a round-bottom flask connected with a vacuum joint. The mixture was degassed in vacuum. The flask was sealed off and placed in an oil bath and polymerized at 130 °C for 48 h. The product was cooled at room temperature, dissolved in chloroform and purified by precipitating into large amount of cold diethyl ether. The precipitated product was vacuum-dried at 40 °C.Synthesis of mPEG–PCL–tosyl and mPEG–PCL–CHS
mPEG–PCL and tosyl chloride were dissolved in CH2Cl2 in an ice bath. TEA was added dropwise in the solution. The solution was stirred at room temperature for 24 h and washed with 0.5 M HCl and saturated brine for three times. The organic phase was dried with anhydrous MgSO4 overnight. The filtrate was concentrated and precipitated in cold anhydrous diethyl ether. The precipitate (mPEG–PCL–tosyl) was vacuum-dried at 40 °C.Chrysin and K2CO3 were dissolved in DMF under nitrogen atmosphere. The mPEG–PCL–tosyl dissolved in DMF was added into the mixture solution with stirring. The reaction was stirred for 12 h at room temperature. The solution was filtrated and the filtrate was concentrated and precipitated in large amount of ethyl ether. The precipitate was dialyzed against deionized water using a 2000 MW cutoff tubing (MWCO 2000, Spectra/Por, USA). The mPEG–PCL–CHS was received after freeze-drying (yield = 90%).
Preparation of drug loaded micelles
The mPEG–PCL–CHS amphiphile (10 mg) and DOX (4.3 mg) were dissolved in 1 mL of DMSO with ultrasound for 0.5 h. The solution was dropped into 10 mL deionized water under strongly stirring overnight. The mixture was dialyzed against deionized water at 4 °C for 12 h in a dialysis membrane tubing (Spectra/Por MWCO = 5000). The solution was removed from the dialysis tubing with centrifugation and lyophilized. The content of DOX was determined by fluorescence measurement (excitation at 485 nm) in DMSO using calibration curve obtained from DOX/DMSO solutions with different DOX concentrations. The whole procedure was processed in dark. The drug loading content (DLC) and encapsulation efficiency (EE) were calculated from the following formulae:| DLC (%) = [weight of drug in micelle/weight of drug loaded micelle] × 100% |
| EE (%) = [weight of drug in micelle/weight of drug in feeding] × 100% |
Release profile
DOX loaded micelles were dispersed in PBS (1 mL, ionic strength = 0.01 M, pH = 7.4). The release experiments were employed under sink conditions in order to ensure the good solubility of DOX. The mixture was put in dialysis membrane tubings (Spectra/Por MWCO = 1000). The tubings were immersed in vials containing 25 mL of phosphate buffered saline (PBS) solution and put in a shaking bed at 37 °C. 1 mL of PBS solution was taken out and the same volume PBS was added to the vials at prescribed time intervals. The released DOX was determined by a fluorescence detector with excitation wavelength at 485 nm and emission wavelength at 550 nm.26,27 The release experiments were conducted in triplicate, the results were demonstrated as mean ± SD.Cytotoxicity test
The cytotoxicity of blank micelles was tested by CCK8 assay against NIH 3T3 fibroblasts and HepG2 cells. The NIH 3T3 fibroblasts and HepG2 cells were separately inoculated into 96-well plates with 5 × 103 cells per well in 100 μL of medium. After 24 h incubation, the medium was removed and replaced with 100 μL of medium containing different concentrations of blank micelles. The micelles were incubated with cells for 48 h. The medium was removed and the wells were washed with PBS (pH = 7.4). 10 μL of 5 mg mL−1 CCK8 solution in PBS (pH = 7.4) was added to each well. After incubated for 4 h, the medium containing unreacted CCK8 was removed carefully and the absorbance was measured.In vitro anticancer activity study
HepG2 cells were separately inoculated into 96-well plates with 5 × 103 cells per well in 100 μL of medium for 24 h. DOX·HCl and DOX loaded micelles solutions in DMEM were added to the plates and incubated for 48 h. The cell viability was measured by CCK8 assay.Cellular uptake
The cellular uptake and distribution of drug loaded micelles in cells were detected using both flow cytometry and confocal laser scanning microscopy (CLSM). For CLSM studies, HepG2 cells were seeded on 35 mm diameter glass dishes at a cell density of 1 × 104 mL−1. HepG2 cells were incubated with DOX·HCl and DOX loaded micelles for 1 h and 3 h (DOX concentration = 10 μg mL−1), the cell nucleus were dyed by Hoechst. The cells were washed by PBS and taken photos under CLSM (Leica TCP SP5). DOX was excited at 480 nm with the emission at 590 nm. For flow cytometry studies, HepG2 cells were seeded on 6-well plates at a density of 2 × 105 cells per well and incubated for 24 h. HepG2 cells were incubated with DOX·HCl and DOX loaded micelles for 1 and 3 h (DOX concentration = 10 μg mL−1). The cells were digested with trypsinization and rinsed with PBS. The cells were resuspended in PBS after centrifugation (1000 rpm, 5 min) and measured for the fluorescence intensity (excitation: 480 nm; emission: 590 nm)24 on a BD FACS Calibur flow cytometer (Beckton Dickinson).Results and discussion
We found that the introduction of π-conjugated moieties in polymeric micelles was favorable for the improvement of stability and drug loading content, and the chrysin modified mPEG–PCL micelles exhibited the optimal properties in anticancer drug delivery. Herein, PCL and mPEG fragments with various molecular weights were used to adjust the properties of the diblock copolymers with the immobilization of chrysin on the terminal group. Four diblock copolymers were fabricated micelles to deliver anticancer drug. The molecular weights of 2000 and 5000 to both mPEG fragments and PCL blocks were used to investigate the chain length effect on drug delivery.The 1H NMR spectra of the four chrysin modified diblock copolymers were shown in Fig. 1. The assignments of the protons in mPEG2k–PCL2k–CHS, mPEG2k–PCL5k–CHS, mPEG5k–PCL2k–CHS and mPEG5k–PCL5k–CHS were presented in Fig. 1. The signals of 1 and 2 at the chemical shifts δ = 3.35 to 4.5 ppm were attributed to the protons in mPEG segments,28 the proton signal in PCL blocks were 3, 4, 5 and 6. The protons from δ = 6.5 to 8 ppm were attributed to CHS (7, 8, 9, 10, 11, 12). As CHS was only immobilized on the terminal group of PCL block, only weak signals of CHS were detected. The molecular weight of the PCL block was calculated by comparing the integrals of characteristic peaks of the PCL block at δ = 2.25 ppm (triplet, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH2–) and mPEG block at δ = 3.35 ppm (singlet, –OCH3).
O)–CH2–) and mPEG block at δ = 3.35 ppm (singlet, –OCH3).
The molecular weights of the four diblock copolymers were also tested by GPC, only one peak was observed in all the GPC spectra (Fig. 2), no unreacted mPEG was observed in the spectra. The calculated molecular weights of both 1H NMR and GPC results were summarized in Table 1. The Mns of mPEG–PCL–CHS copolymers calculated from 1H NMR spectra were comparable to the designed molecular weights. As the molecular weight tested by GPC was the relative molecular weight to the polystyrene standard samples, the Mns tested by GPC were deviated away from the theoretical values. It was interesting that the copolymers with mPEG5k blocks showed very narrow polydispersities. The PDIs of mPEG5k–PCL2k–CHS and mPEG5k–PCL5k–CHS copolymers were 1.06 and 1.05, respectively.
| Sample | Molecular weight | Micelle diameter (nm) | DLC (%) | EE (%) | |||
|---|---|---|---|---|---|---|---|
| Mna | Mnb | Mw/Mn | Blank | Drug loaded | |||
| a Calculated from 1H NMR spectra.b Tested by GPC. | |||||||
| mPEG2k–PCL2k–CHS | 4800 | 6700 | 1.14 | 15.5 ± 1.1 | 56.6 ± 9.1 | 20.9 ± 0.7 | 80.0 ± 0.9 |
| mPEG2k–PCL5k–CHS | 7500 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.38 | 25.3 ± 2.8 | 106.5 ± 6.8 | 26.8 ± 0.9 | 93.0 ± 0.8 |
| mPEG5k–PCL2k–CHS | 7100 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.06 | 30.3 ± 2.0 | 60.4 ± 6.4 | 21.1 ± 0.6 | 85.0 ± 0.3 |
| mPEG5k–PCL5k–CHS | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.05 | 39.4 ± 1.6 | 61.3 ± 4.9 | 24.5 ± 0.9 | 87.0 ± 0.6 |
The crystallization of the four diblock copolymers was investigated by DSC and XRD (Fig. 3). Fig. 3A presented clear melting endothermal peaks in the thermograms of mPEG–PCL–CHS block copolymers with different chain length. The melting temperatures (Tms) of mPEG2k–PCL2k–CHS, mPEG2k–PCL5k–CHS, mPEG5k–PCL2k–CHS and mPEG5k–PCL5k–CHS copolymers were 48.4, 43.0 (51.8), 54.7 and 55.5 °C, and the ΔHs were 106.0, 69.0, 114.5 and 105.8 J g−1. The Tms of mPEG and PCL blocks were nearly the same around 50 °C,15 it was hard to observe the respective melting endothermal peaks of mPEG and PCL except mPEG2k–PCL5k–CHS. In the thermogram of mPEG2k–PCL5k–CHS, two melting points of 43.0 and 51.8 °C appeared, which were attributed to the blocks of mPEG2k and PCL5k, respectively. As we knew that the Tm of semi-crystal polymer commonly increased with increasing molecular weight. The discrepancy of Tm between mPEG2k and PCL5k was large enough to be observed in the thermogram of mPEG2k–PCL5k–CHS. The crystals of mPEG and PCL blocks were mixed together in mPEG–PCL–CHS and they could not be separated in DSC results. XRD was an effective tool to discover the crystals of mPEG and PCL blocks. In the XRD spectra of the four copolymers (Fig. 3B), the characteristic crystal peaks of PCL blocks at 2θ = 21.5° and 23.8° were obvious, mPEG showed two strong peaks at 2θ = 19.1° and 23.4°.28 The peaks at 23.4 and 23.8 degree were overlapped to exhibit a strong peak. The crystallinities calculated from XRD spectra were 72.3%, 60.3%, 76.0% and 70.3% for mPEG2k–PCL2k–CHS, mPEG2k–PCL5k–CHS, mPEG5k–PCL2k–CHS and mPEG5k–PCL5k–CHS copolymers, which was consistent with the ΔHs in DSC results.
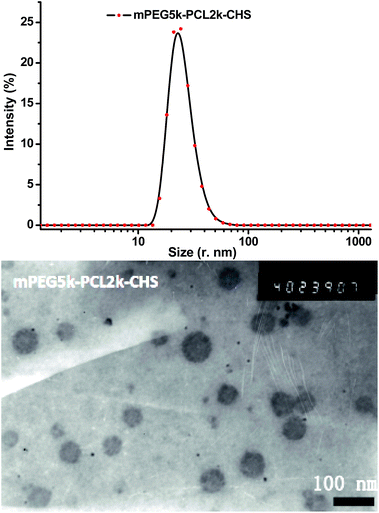
The size and morphology of mPEG–PCL–CHS micelles were tested by DLS and TEM (Fig. 4). The polymeric micelles were monodisperse in DLS result. TEM image of micelles showed the micelles were in spherical shape. The sizes of the four mPEG–PCL–CHS micelles tested by DLS were summarized in Table 1. The mean sizes of mPEG2k–PCL2k–CHS, mPEG2k–PCL5k–CHS, mPEG5k–PCL2k–CHS and mPEG5k–PCL5k–CHS micelles were 15.3, 25.3, 30.3 and 39.4 nanometers. Anticancer drug doxorubicin was encapsulated in the mPEG–PCL–CHS micelles, the mean sizes of the four drug loaded micelles were 56.6, 106.5, 60.4 and 61.3 nanometers, which were enlarged after drug encapsulation. Drug loading content and encapsulation efficiency were also measured, the DLCs of all the four micelles were higher than 20%. All the encapsulation efficiencies were higher than 85%. Within all the four species, mPEG2k–PCL5k–CHS micelle exhibited the highest DLC and EE, it possessed the largest mean size probably due to the highest DLC within all the drug loaded micelles. The mean diameters of both blank and drug loaded micelles in TEM images were calculated, the results were consistent with those of DLS results.
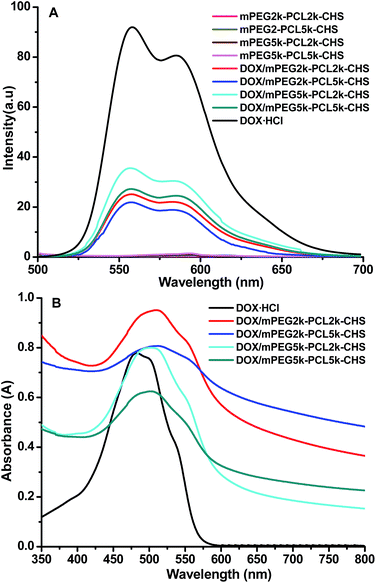
The interaction between DOX and mPEG–PCL–CHS micelles was investigated. The π–π stacking interaction within the drug loaded micelles was tested by fluorescence spectra and UV-vis absorption (Fig. 5). All the four DOX/mPEG2k–PCL2k–CHS, DOX/mPEG2k–PCL5k–CHS, DOX/mPEG5k–PCL2k–CHS and DOX/mPEG5k–PCL5k–CHS micelles exhibited remarkable decrease in the fluorescence intensity of emission band with the same exciting wavelength of 485 nm and DOX concentration of 50 μg mL−1, respectively. The significant quenching was the evidence of π-stacked DOX,29,30 which indicated that the DOX was packed into the micelles. The DOX/mPEG2k–PCL5k–CHS exhibited the greatest extent quenching and the minimum quenching appeared in DOX/mPEG5k–PCL2k–CHS. The more quenching extent in fluorescence revealed the stronger π–π stacking interaction within DOX loaded micelles.19 Red shift was observed in DOX loaded micelles (Fig. 5B). The red shift of DOX was generally due to π–π stacking or ground-state electron donor–acceptor interaction between DOX and carriers.24 The drug loaded micelles with longer PCL chain length showed wider red shift.
mPEG–PCL micelles were extensively reported as carriers to deliver anticancer drugs, they were non-toxic to cells. In order to investigate the cytotoxicity of chrysin modified mPEG–PCL micelles, NIH/3T3 fibroblasts and HepG2 liver cancer cells were incubated with mPEG–PCL–CHS micelles with different concentrations. The cell viabilities of the two cell lines were presented in Fig. 6, the cell viabilities of mPEG–PCL–CHS micelles to both NIH/3T3 and HepG2 cells were higher than 90% even the concentration of micelles was as high as 500 μg mL−1, which was much higher than the concentration applied in vitro and in vivo. The results revealed that the four mPEG–PCL–CHS micelles were non-toxic to cells.
 | ||
| Fig. 6 Cytotoxicity of mPEG–PCL–CHS micelles incubated with 3T3 fibroblasts (A) and HepG2 cancer cells (B). | ||
The in vitro release profiles of DOX loaded micelles were carried out in physiological condition (pH = 7.4) at 37 °C. The burst release was found in all the four samples in the first 10 hours, however, the extent of burst release was different. The release rate decreased with the sequence of mPEG5k–PCL2k–CHS, mPEG5k–PCL5k–CHS, mPEG2k–PCL2k–CHS and mPEG2k–PCL5k–CHS. DOX/mPEGk–PCL5k–CHS exhibited the best profile in sustained release (Fig. 7A). The drug release mechanism in these polymeric micelles was diffusion control. The drug diffused from the hydrophobic cores of micelles to the medium. The burst release was attributed to the drug absorbed in the hydrophilic PEG layer, which diffused easier and faster to the medium. Interestingly, the release rate sequence of drug loaded micelles was consistent with the sequence of fluorescence quenching in Fig. 5A, it supported the conclusion that more extent quenching implied stronger π–π stacking interaction between DOX and micelles, which prevented the release of DOX from micelles and resulted in low drug release rate. The release profiles indicated that the sustained release of DOX was achieved and the drug release rate could be controlled by modulating the chain length of mPEG and PCL blocks.15
 | ||
| Fig. 7 The release profiles (A) and inhibition effect (B) of DOX loaded mPEG–PCL–CHS micelles to HepG2 cells. | ||
The DOX loaded micelles were incubated with HepG2 cells to evaluate the in vitro anticancer activity (Fig. 7B). The in vitro anticancer efficiency of DOX loaded micelles was dose-dependent. The IC50s (the concentration of anti-drug that killed 50% of cells) of free DOX·HCl, DOX/mPEG2k–PCL5k–CHS, DOX/mPEG2k–PCL2k–CHS, DOX/mPEG2k–PCL5k–CHS and DOX/mPEG5k–PCL2k–CHS micelles were 0.1, 0.25, 0.42, 0.65 and 2.04 μg mL−1, respectively. As water-soluble anticancer drug, DOX·HCl diffused into cells and chelated into the DNA backbone to destroy DNA replication,25 the diffusion of DOX·HCl was faster in cellular internalization to the endocytosis of drug loaded micelles. The IC50s of the four drug loaded micelles were coincident with the release profile. Lower drug release rate was corresponded to lower IC50, DOX/mPEG2k–PCK5k–CHS micelles exhibited the best anticancer efficiency.
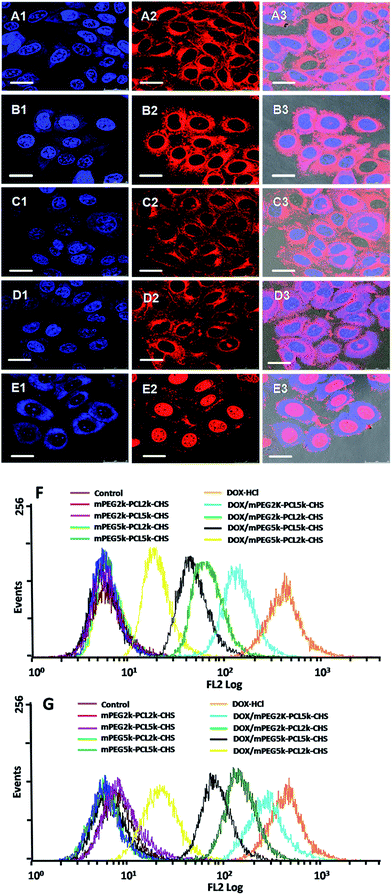
The intracellular localization and distribution of DOX·HCl and DOX loaded micelles were investigated against HepG2 cells using both confocal fluorescence microscopy and flow cytometry (Fig. 8). The HepG2 cells treated with DOX·HCl showed that strong red fluorescence intensity was mainly in nucleus and emitted weakly in the surrounding cytoplasm after 4 h incubation. It was because that free DOX·HCl was internalized into cells via a passive diffusion mechanism.31,32 The affiliation between DNA and DOX was strong and thus free DOX·HCl mainly located in nuclei. The cellular uptake of DOX loaded micelles was clearly visible, the red fluorescence of DOX was scattered intensely in cytosolic compartments. This result showed that the DOX loaded micelles were internalized via an endocytosis process, which was different from that of DOX·HCl, the encapsulated DOX could escape from endosomes and/or lysosomes to fulfill anticancer function.24 With the comparison of DOX loaded micelles, the evidently higher intracellular DOX fluorescence intensity was observed in HepG2 cells incubated with DOX/mPEG2k–PCL5k–CHS.
HepG2 cells without any treatment and with the treatment of blank micelles were used as the negative controls in flow cytometry test (Fig. 8F and G), there was only autofluorescence of the cells, which was not changed with time extension. The fluorescence intensity of DOX·HCl was the highest, and the following sequence was DOX/mPEG2k–PCL5k–CHS, DOX/mPEG2k–PCL2k–CHS, DOX/mPEG5k–PCL5k–CHS and DOX/mPEG5k–PCL2k–CHS. With longer incubation time (3 h), HepG2 cells treated with DOX/mPEG2k–PCL5k–CHS still showed the strongest fluorescence within all the four drug loaded micelles. The flow cytometry results were in agreement with the results of confocal fluorescence microscopy study.
Conclusions
Four amphiphilic mPEG–PCL copolymers with different chain length were synthesized via ring-opening polymerization of ε-CL with mPEG as macroinitiator, the terminal group of PCL segment was modified with chrysin to introduce π–π stacking interaction with anticancer drug doxorubicin. The chain length effects of chrysin modified mPEG–PCL diblock copolymers on the crystallization behavior of copolymers, self-assembly of micelles, drug loading, release profiles, cellular uptake and in vitro anticancer activity of drug loaded micelles were investigated. All the four micelles exhibited promising drug loading content higher than 20%, the mPEG2k–PCL5k–CHS micelles showed the highest drug loading content and encapsulation efficiency of 26.8% and 93%, respectively. The micelles were spherical with the size around 100 nanometers. The π–π stacking interaction between micelles and doxorubicin was evoked after drug encapsulation. The release rate decreased in the sequence of drug loaded mPEG5k–PCL2k–CHS, mPEG5k–PCL5k–CHS, mPEG2k–PCL2k–CHS and mPEG2k–PCL5k–CHS micelles. DOX/mPEGk–PCL5k–CHS exhibited the best profile in sustained release. The results of cellular uptake and IC50 revealed that the mPEG2k–PCL5k–CHS micelles exhibited the best in vitro anticancer activity.Acknowledgements
The authors thank for the financial support of National Science Foundation of China (No. 51222304, 31170921, 51403137), Ministry of Education of China (No. 20130181110038), Sichuan University (No. 2014SCU11015), Fundamental Research of Qingdao Science & Technology Program (No. 13-1-4-231-jch).Notes and references
- K. Kawano, M. Watanabe, T. Yamamoto, M. Yokoyama, P. Opanasopit, T. Okano and Y. Maitani, J. Controlled Release, 2006, 112, 329–332 CrossRef CAS PubMed.
- C. Yang, A. B. Ebrahim Attia, J. P. K. Tan, X. Ke, S. Gao, J. L. Hedrick and Y.-Y. Yang, Biomaterials, 2012, 33, 2971–2979 CrossRef CAS PubMed.
- M. Talelli, M. Barz, C. J. F. Rijcken, F. Kiessling, W. E. Hennink and T. Lammers, Nano Today, 2015, 10, 93–117 CrossRef CAS PubMed.
- S. W. Kang, Y. Li, J. H. Park and D. S. Lee, Polymer, 2013, 54, 102–110 CrossRef CAS PubMed.
- F. R. Cheng, Y. J. Yang, Y. Liang, J. Q. Yan, J. Cao, T. Su, L. Jiang, B. He, X. L. Luo and Z. W. Gu, RSC Adv., 2014, 4, 62708–62716 RSC.
- H. Park, J. Yang, J. Lee, S. Haam, I.-H. Choi and K.-H. Yoo, ACS Nano, 2009, 3, 2919–2926 CrossRef CAS PubMed.
- L. G. Lis, M. A. Smart, A. Luchniak, M. L. Gupta and V. J. Gurvich, ACS Med. Chem. Lett., 2012, 3, 745–748 CrossRef CAS PubMed.
- J. Liu, Z. Wang, K. Wu, J. Li, W. Chen, Y. Shen and S. Guo, Biomaterials, 2015, 53, 592–599 CrossRef CAS PubMed.
- F. M. Menger, H. Lu and D. Lundberg, J. Am. Chem. Soc., 2007, 129, 272–273 CrossRef CAS PubMed.
- Q. Hu, C. J. Rijcken, R. Bansal, W. E. Hennink, G. Storm and J. Prakash, Biomaterials, 2015, 53, 370–378 CrossRef CAS PubMed.
- A. Dirisala, K. Osada, Q. Chen, T. A. Tockary, K. Machitani, S. Osawa, X. Liu, T. Ishii, K. Miyata, M. Oba, S. Uchida, K. Itaka and K. Kataoka, Biomaterials, 2014, 35, 5359–5368 CrossRef CAS PubMed.
- M. A. Moretton, R. J. Glisoni, D. A. Chiappetta and A. Sosnik, Colloids Surf., B, 2010, 79, 467–479 CrossRef CAS PubMed.
- J. C. Cuggino, M. Calderón, C. I. Alvarez, M. C. Strumia, K. N. Silva, E. K. Penott-Chang and A. J. Müller, J. Colloid Interface Sci., 2011, 357, 147–156 CrossRef CAS PubMed.
- Y. Liang, Y. Lai, D. Li, B. He and Z. Gu, Mater. Lett., 2013, 97, 4–7 CrossRef CAS PubMed.
- Y. Zhang and R. Zhuo, Biomaterials, 2005, 26, 6736–6742 CrossRef CAS PubMed.
- W. Li, M. Nakayama, J. Akimoto and T. Okano, Polymer, 2011, 52, 3783–3790 CrossRef CAS PubMed.
- S. H. Kim, J. P. K. Tan, K. Fukushima, F. Nederberg, Y. Y. Yang, R. M. Waymouth and J. L. Hedrick, Biomaterials, 2011, 32, 5505–5514 CrossRef CAS PubMed.
- Q. Hu, E. V. B. van Gaal, P. Brundel, H. Ippel, T. Hackeng, C. J. F. Rijcken, G. Storm, W. E. Hennink and J. Prakash, J. Controlled Release, 2015, 205, 98–108 CrossRef CAS PubMed.
- X. Deng, Y. Liang, X. Peng, T. Su, S. Luo, J. Cao, Z. Gu and B. He, Chem. Commun., 2015, 51, 4271–4274 RSC.
- H. Zheng, S. Li, Y. Pu, Y. Lai, B. He and Z. Gu, Eur. J. Pharm. Biopharm., 2014, 87, 454–460 CrossRef CAS PubMed.
- Y. Lei, Y. Lai, Y. Li, S. Li, G. Cheng, D. Li, H. Li, B. He and Z. Gu, Int. J. Pharm., 2013, 453, 579–586 CrossRef CAS PubMed.
- D. Li, Y. Liang, Y. Lai, G. Wang, B. He and Z. Gu, Colloids Surf., B, 2014, 116, 627–632 CrossRef CAS PubMed.
- Y. Li, T. Su, S. Li, Y. Lai, B. He and Z. Gu, Biomater. Sci., 2014, 2, 775–783 RSC.
- Y. Lai, Y. Lei, X. Xu, Y. Li, B. He and Z. Gu, J. Mater. Chem. B, 2013, 1, 4289–4296 RSC.
- H. Yoo, J. Oh, K. Lee and T. Park, Pharm. Res., 1999, 16, 1114–1118 CrossRef CAS.
- R. Liu, D. Li, B. He, X. Xu, M. Sheng, Y. Lai, G. Wang and Z. Gu, J. Controlled Release, 2011, 152, 49–56 CrossRef CAS PubMed.
- D. Kim, E. Lee, K. Park, I. Kwon and Y. Bae, Pharm. Res., 2008, 25, 2074–2082 CrossRef CAS PubMed.
- I. L. Gyun Shin, S. Yeon Kim, Y. Moo Lee, C. Soo Cho and S. Yong Kiel, J. Controlled Release, 1998, 51, 1–11 CrossRef.
- Z. Liu, X. Sun, N. Nakayama-Ratchford and H. Dai, ACS Nano, 2007, 1, 50–56 CrossRef CAS PubMed.
- N. Nakayama-Ratchford, S. Bangsaruntip, X. Sun, K. Welsher and H. Dai, J. Am. Chem. Soc., 2007, 129, 2448–2449 CrossRef CAS PubMed.
- Y. Lee, S. Y. Park, H. Mok and T. G. Park, Bioconjugate Chem., 2008, 19, 525–531 CrossRef CAS PubMed.
- Y. Sun, X. Yan, T. Yuan, J. Liang, Y. Fan, Z. Gu and X. Zhang, Biomaterials, 2010, 31, 7124–7131 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |