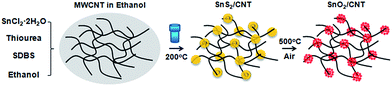
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbon nanotube-assisted growth of single-/multi-layer SnS2 and SnO2 nanoflakes for high-performance lithium storage†
Dongsheng
Guan
,
Jianyang
Li
,
Xianfeng
Gao
and
Chris
Yuan
*
Department of Mechanical Engineering, University of Wisconsin Milwaukee, Milwaukee, USA 53211. E-mail: cyuan@uwm.edu; Fax: +1 414 229 5639; Tel: +1 414 229 6958
First published on 29th June 2015
Abstract
SnS2 nanoparticles and SnS2 nanoflake/CNTs composite are prepared by a low-cost facile hydrothermal method for their use in rechargeable Li-ion batteries. It is found that the presence of multi-walled CNTs during synthesis greatly affects the morphology of as-formed SnS2 nanostructures, and circinal single-layer and multilayer SnS2 nanoflakes enwrapped by CNTs are produced. The composite is further oxidized to porous SnO2 nanoflake/CNTs hybrid by annealing at 500 °C in air. The formation mechanism of SnS2/CNTs and SnO2/CNTs composites is examined. All the three materials are used as the anode in Li-ion batteries. The SnS2/CNTs composite delivers stronger cycling stability than the pure SnS2 anode. In tests the former exhibits excellent capacity retention of 91.5% at 100 mA g−1 over 50 cycles, while the latter displays 66.8%. The rate capability of SnS2/CNTs composite is much better than pure SnS2 as well. Redox reaction characteristics and Li-ion transfer kinetics at the two SnS2 anodes are studied by differential capacity plots and electrochemical impedance spectroscopy. It is discovered that the SnS2/CNTs composite with larger surface area allows faster Li-ion transfer kinetics, effective cushion of volume changes, and thus gains the improved Li-ion intercalation behaviours. The capacity of the tin-based anode can be further raised by transformation to a SnO2/CNTs hybrid that also delivers excellent rate and cycling performances.
1. Introduction
Currently, the soaring development of electronic devices and electric vehicles urgently demands novel mobile power sources with high energy and power density, good safety and durability. Rechargeable Li-ion batteries (LIBs) have become a candidate due to their large energy storage density, light weight, low self-discharge and long cycle life, no memory effects.1,2 Graphite is the most commonly-used anode material in current commercial LIBs, but the low capacity and poor safety of graphite fail to meet the demand of next-generation LIBs, so a group of novel anode materials have been developed to replace it, including metal sulphides and oxides.3–6 Among them, tin and tin-based materials such as tin alloys, tin oxides and tin sulphides are considered as potential anode materials for LIBs mostly owing to their large theoretical capacity and good reversibility. Tin disulphide (SnS2) is an extensively-studied anode material with a layered hexagonal CdI2-type structure, in which sheets of tin atoms are sandwiched between two close packed sheets of sulphur atoms, and they are bound by weak van der Waals forces.7 A group of SnS2 nanostructures, such as nanoparticles,8 nanoplates,9 nanosheets,10 nanobelts,11 nanoflower and nanorods,12,13 have been synthesized by methods including hydrothermal and solvothermal methods, thermal sulfurization, wet chemistry and vapor deposition. In the practice it is found that SnS2 with a theoretical capacity of 645 mA h g−1 suffers a large volume change (≥200%) and particle aggregation related to Li-ion insertion/extraction process, which leads to electrode pulverization and a loss of effective contact between the active material and in turn results in drastic capacity decay and poor cyclability.14 In addition, low conductivity of SnS2 (10−12–10−2 S cm−1) highly impedes electronic transfer and deteriorates its rate performance. A number of approaches are proposed to alleviate these problems. Among them, carbonaceous materials are often used to enhance the electrochemical performance of SnS2. Kim et al. achieved carbon-coated SnS2 nanoparticles that show far better Li-ion intercalation behaviour than uncoated SnS2.15 He et al. synthesized acetylene black incorporated SnS2 nanoflower with increased Li-ion storage capacities.16 Zhai et al. produced SnS2 nanosheet anchored MWCNTs and found that the hybrid delivers higher capacity and cyclability than both the MWCNTs and SnS2.17 Sun et al. also fabricated SnS2 nanoflake decorated MWCNTs with improved capacity and cycling performance.18 Kang et al. discovered self-supported SnS2 nanosheet coated MWCNTs with higher capacity and capability than pure SnS2 nanosheets.19 Tin dioxide (SnO2) is an attractive anode material as well, due to its high theoretical capacity of 781 mA h g−1, but its huge volume change during Li-ion storage process results in a big loss of reversible capacity. Therefore, various methods are invented to solve this problem, such as SnO2 coatings on TiO2–B nanowires,20 CNTs and graphene.21,22 Those carbon materials not only give birth to new SnS2 or SnO2 nanostructures but also favor charge transfer and electrode stability, yielding improved Li-ion battery performances. So far, a new type of composites consisting of two-dimensional (2D) electrode materials and CNTs network has been reported, including MoS2 nanosheet/MWCNTs,3 CoOOH nanoplate/MWCNTs and Ni(OH)2 nanoplate/MWCNTs.23,24 The nanosheets or nanoplates embed into the porous matrix and connect conducting MWCNTs to achieve enhanced redox reaction kinetics and electronic conductivity, and finally obtain improved electrochemical properties. To the best of our knowledge, the composite composed of 2D SnS2 or SnO2 nanoflakes dispersed in such a CNTs network for LIBs applications has not been reported hitherto. We consider that CNTs can not only improve the conductivity of SnS2 or SnO2, but cushion the volume change and retard the agglomeration of the active material, which is possible to achieve novel tin-based anodes with high capacity, good cyclability and rate capability.In the present work, we synthesized SnS2 nanoparticles, SnS2 nanoflake/CNTs and SnO2 nanoflake/CNTs hybrids by scalable hydrothermal method and annealing. MWCNTs were found to promote the 2D growth of single-/multi-layer SnS2 nanoflakes, improve the ionic and electronic transfer of electrodes. The thin nanoflakes can minimize the volume change during cycling for better cyclability and provide more sites to accommodate Li-ion insertion for increased capacities. A post heat treatment of these SnS2/CNTs composite in air yielded a new SnO2/CNTs hybrid that inherited the porous, nanoflake-like features, and exhibited further increased specific capacities.
2. Results and discussion
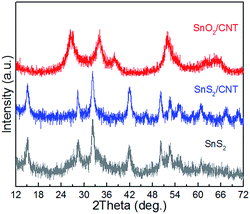
Fig. 1 shows XRD patterns of as-prepared SnS2, SnS2/CNTs and SnO2/CNTs composites. In the pattern of SnS2 diffraction peaks (001), (100), (101), (102), (110), (111), (103), (200), (201), (202) and (113) of hexagonal SnS2 (JCPDS no. 23-0677, space group P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1) are observed at the 2θ angles of 15.1°, 28.3°, 32.2°, 41.9°, 50.1°, 52.5°, 55°, 58.5°, 60.9°, 67.2° and 70.4°, which demonstrates good crystallization of SnS2 during hydrothermal process. The XRD pattern of SnS2/CNTs hybrid is almost the same as that of SnS2, with no peaks corresponding to MWCNTs, but the SnO2/CNTs composite gives a different pattern. Typical peaks at the 2θ angle of 26.6°, 33.9°, 38°, 51.9°, 54.7°, and 64.8° are indexed to (110), (101), (200), (211), (220) and (112) crystal planes of tetragonal SnO2 (JCPDS no. 41-1445, space group P42/mnm). Again, no diffraction peaks that belong to MWCNTs are identified from the pattern of SnO2/CNTs. It is noted that the characteristics of XRD patterns of SnS2 and SnO2 (an oxidation product of SnS2) here are in agreement with phase change described in previous reports.25,26
m1) are observed at the 2θ angles of 15.1°, 28.3°, 32.2°, 41.9°, 50.1°, 52.5°, 55°, 58.5°, 60.9°, 67.2° and 70.4°, which demonstrates good crystallization of SnS2 during hydrothermal process. The XRD pattern of SnS2/CNTs hybrid is almost the same as that of SnS2, with no peaks corresponding to MWCNTs, but the SnO2/CNTs composite gives a different pattern. Typical peaks at the 2θ angle of 26.6°, 33.9°, 38°, 51.9°, 54.7°, and 64.8° are indexed to (110), (101), (200), (211), (220) and (112) crystal planes of tetragonal SnO2 (JCPDS no. 41-1445, space group P42/mnm). Again, no diffraction peaks that belong to MWCNTs are identified from the pattern of SnO2/CNTs. It is noted that the characteristics of XRD patterns of SnS2 and SnO2 (an oxidation product of SnS2) here are in agreement with phase change described in previous reports.25,26
Fig. 2 presents SEM images of SnS2, SnS2/CNTs and SnO2/CNTs materials. It is clearly seen that the absence of MWCNTs yields a big number of SnS2 nanoparticles mostly with irregular shape and size (Fig. 2a). Some particles display an appearance of hexagonal nanoplates, indicating non-uniform growth of SnS2 nanostructures under the hydrothermal conditions. An enlarged view of one SnS2 nanoplate reveals its lateral size up to 650 nm and thickness up to ∼100 nm (Fig. 2b). The presence of MWCNTs during synthesis leads to formation of a distinct nanostructure. As shown in Fig. 2c, considerable 2D SnS2 nanoflakes are enwrapped by MWCNTs to form a porous SnS2/CNTs hybrid structure. The nanoflakes are dense and thin, with a single layer or multiple layers. The multilayer nanoflake is constructed by small flakes developing around the centre of large flakes or underneath (Fig. 2d and S1a†). MWCNTs either insert into the flakes parallel or spread over them to bundle nanoflakes into their conductive network, rather than being coated or anchored with SnS2.17,18 The flakes display more uniform shapes, with a diameter up to 600 nm and a thickness of 10 nm (Fig. S1b†), which claims that SnS2 prefers 2D growth mode along neighbouring CNTs when the two coexist in the superhot solution system. It is believed that the structure of 2D nanoflakes and loose CNTs network can possess larger specific surface than SnS2 nanoparticles that are large and aggregated. In fact, BET measurements demonstrate that the surface area is 15.48 m2 g−1 for pure SnS2 and 47.87 m2 g−1 for the SnS2/CNTs (twice larger) (Fig. S2†). After annealing, SnS2 is oxidized to be thin SnO2 nanoflakes enwrapped in the CNT network (Fig. 2e). The nanoflake/CNTs structure is preserved, but the nanoflakes become porous and rough, which contain numerous tiny SnO2 crystallites (Fig. 2f). Hence it is deduced that the oxide particles are supposed to originate from SnS2 crystals and connect with each other to maintain the nanoflake-like shape. Excellent Li-intercalation behaviours of the two composites are expected, because the conductive MWCNTs, thin nanoflakes and porous structure are considered to endow fast charge transfer and small electrode resistance.23,24
 | ||
| Fig. 2 SEM images of (a and b) pure SnS2 particles, (c and d) SnS2/CNTs composite and (e and f) SnO2/CNTs composite. | ||
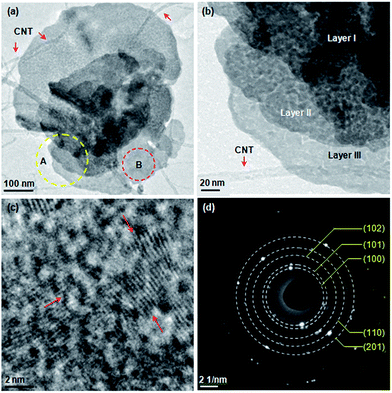
The two nanoflake/CNTs composites are further observed by TEM. Fig. 3a shows a SnS2 nanoflake conjunct CNTs. It has multiple layers, with several slim CNTs across its surface or underneath. An enlarged TEM image reveals that all the three-layer flakes are actually composed of nanoparticles (Fig. 3b). These particles are SnS2 polycrystallites clearly seen in Fig. 3c. A selected area electron diffraction ring pattern of this part shows multiple indices (100), (101), (102), (110) and (201) of SnS2 (Fig. 3d), which agrees well with the XRD index above.
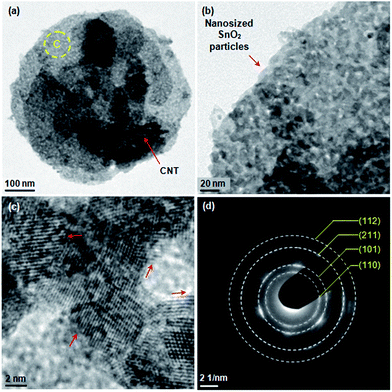
Upon annealing SnS2 is oxidized and a SnO2/CNTs composite results. Both the nanoflake and CNTs survive (Fig. 4a). The enlarged view of spot C in Fig. 4a shows that the nanoflake is also composed of considerable nanoparticles (Fig. 4b), which is corresponding to our SEM observation above. They are SnO2 polycrystallites with a size of ∼5 nm (Fig. 4c). The selected area electron diffraction ring patterns reveals the (110), (101), (211) and (112) crystal planes of SnO2 in this nanoflake (Fig. 4d).
EDS analyses were performed on the SnS2/CNTs composite under SEM observation. In the spectrum in Fig. 5, distinct peaks between 3.3 and 4.1 keV correspond to the tin element. Sulfur peak appears at a position of 2.4 keV. Carbon is also found in this pattern, which is believed to be mostly from CNTs. A small peak belonging to oxygen is found, indicative of existence of oxygen species due to acid treatment. Al peak is from the holder used for placing the sample during observation. A linear scan of a multilayer nanoflake vividly tells that tin and sulphur are dominant elements there with a negligible amount of carbon and oxygen (Fig. 5b), which is corresponding to the aforementioned XRD pattern of the SnS2/CNTs sample. After annealing in air, SnS2 in the composite is completely oxidized to SnO2, as evidenced by the disappearance of sulphur peak and the birth of stronger oxygen peak in the EDS spectrum of the SnO2/CNTs composite (Fig. S3†).
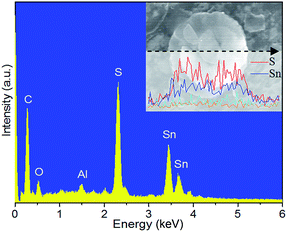 | ||
| Fig. 5 EDS spectrum of SnS2/CNTs composite. The inset is elemental linear scan across a single SnS2 nanoflake. | ||
The composition of two composites is further investigated by XPS technique. As for the SnS2/CNTs sample, the peaks at 486.86, 495.26 and 161.7 eV in the high-resolution XPS spectra (Fig. 6a and b) are assigned to Sn 3d5/2, Sn 3d3/2 and S 2p, respectively. The energy difference between two tin peaks is 8.4 eV, indicating the existence of Sn(IV). The results confirm the SnS2 compound in the composite. The broad C 1s peak at 284.8 eV is asymmetric (Fig. 6c), which is consisting of four subpeaks at 283.8, 284.3, 285.4 and 288.4 eV (Fig. S4a†). The peak at 283.8 and 284.3 eV could be assigned to sp2 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and sp3 C–C bond, either in a graphite phase or in an amorphous phase.16 Peaks at 285.4 and 288.4 eV indicate the existence of residual groups such as –OH and –O–C
C bond and sp3 C–C bond, either in a graphite phase or in an amorphous phase.16 Peaks at 285.4 and 288.4 eV indicate the existence of residual groups such as –OH and –O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, probably due to the acid treatment of CNTs and the adsorbed reactant.16 Likewise, Sn(IV) is detected in the SnO2/CNTs composite but sulphur not (Fig. 6d). It can be seen in Fig. 6b that broad O 1s peak at 531.3 eV is composed of three subpeaks at 530.5 eV (Sn–O bond), 531.6 eV (Sn–O–C bond) and 532.9 eV (C–O bond)27 (Fig. S4b†). C 1s XPS peak from the SnO2/CNTs is asymmetric as well at 284.6 eV (Fig. 6f), which includes three subpeaks at 283.8 eV (C
O, probably due to the acid treatment of CNTs and the adsorbed reactant.16 Likewise, Sn(IV) is detected in the SnO2/CNTs composite but sulphur not (Fig. 6d). It can be seen in Fig. 6b that broad O 1s peak at 531.3 eV is composed of three subpeaks at 530.5 eV (Sn–O bond), 531.6 eV (Sn–O–C bond) and 532.9 eV (C–O bond)27 (Fig. S4b†). C 1s XPS peak from the SnO2/CNTs is asymmetric as well at 284.6 eV (Fig. 6f), which includes three subpeaks at 283.8 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond), 284.6 eV (C–C bond), 285.3 eV (C–O bond) and 288.4 eV (O–C
C bond), 284.6 eV (C–C bond), 285.3 eV (C–O bond) and 288.4 eV (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond) as shown in Fig. S4c.† Thereby, it can be easily concluded that SnS2 and SnO2 nanoflakes have been successfully produced and incorporated into the CNTs network by hydrothermal means and annealing in oxidative atmosphere.
O bond) as shown in Fig. S4c.† Thereby, it can be easily concluded that SnS2 and SnO2 nanoflakes have been successfully produced and incorporated into the CNTs network by hydrothermal means and annealing in oxidative atmosphere.
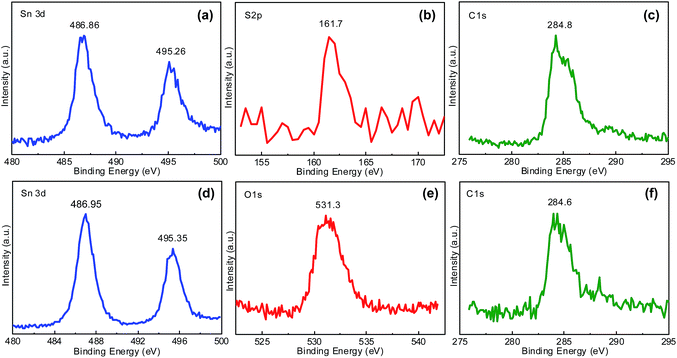 | ||
| Fig. 6 XPS spectra of (a) Sn 3d, (b) S 2p, (c) C 1s signals taken from SnS2/CNTs composite, of (d) Sn 3d, (e) O 1s and (f) C 1s signals taken from SnO2/CNTs composite. | ||
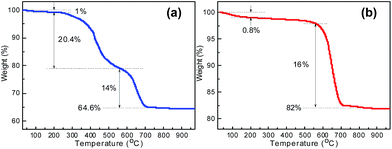
The percentages of CNTs in the SnS2/CNTs and SnO2/CNTs composites were determined by TG analysis in air atmosphere, as shown in Fig. 7. The data presented in Fig. 7a reveals that the TG plot of SnS2/CNTs hybrid shows a three-step mass-loss process. A small weight loss of ∼1% from room temperature to 200 °C can be attributed to the removal of physically adsorbed water. The next weight loss step between 200 °C and 560 °C is ascribed to the oxidation of SnS2 into SnO2.18 The third weight loss observed from 560 °C to 800 °C is owing to the burning of CNTs in the composite.28 The total weight loss suffered by the composite is 35.4%. As for the SnO2/CNTs hybrid, the drastic weight loss of 16% observed from 560 °C to 800 °C results from the burning of MWCNTs (Fig. 7b). This confirms that the second weight loss of SnS2/CNTs hybrid is truly owing to the oxidation of SnS2 to SnO2. As a result, the weight fraction of CNTs in the SnS2/CNTs and SnO2/CNTs is estimated to be ca. 14% and 16%, respectively. The high ratio of CNTs is likely to contribute to the advantage of constructing conductive network for enhanced tin-based anode performance.
Based on above analyses, the probable growth process of the two composites is discussed with schematic illustration shown in Fig. 8. The formation of tin sulfide (SnS) precursors may be described as follows:12
| Sn2+ + 2C2H5OH → 2Sn(C2H5O)2 + 2H+ | (1) |
| NH2CSNH2 + 4C2H5OH → 2NH3↑ + 2C2H5OC2H5 + CO2 + H2S | (2) |
| H2S + Sn(C2H5O)2 → SnS + 2C2H5OH | (3) |
It is noted that eqn (2) takes place in the presence of H+ in the superhot solution.12 We know that increasing the temperature encourages the oxidation reaction of Sn2+ to Sn4+; when the temperature goes up to 140 °C and more, SnS2 originates from SnS.29 Thus, we speculate that SnS forms initially and it is then oxidized to SnS2 in the aid of excessive sulfur sources. Hence, the formation of many SnS2 nuclei occurs in the hydrothermal process. They grow and start to agglomerate with each other. The further growth of these agglomerates is restricted fairly by dispersed CNTs, so they prefer to develop parallel to the CNTs, eventually yielding uniform thin nanoflakes inside the CNTs network (Fig. 8). Upon heat treatment in air, SnS2 nanoflakes are oxidized in situ to form SnO2 nanoflakes in the network.
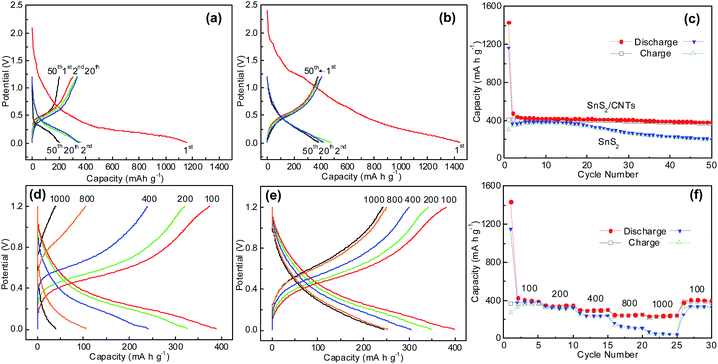
In this study the Li-ion intercalation behaviour of SnS2 and SnS2/CNTs hybrids are first compared to investigate the effects of MWCNTs on the electrochemical property. The potential of redox reactions is studied via charge–discharge plots measured at 100 mA g−1 (Fig. 9a and b). Both electrodes have large initial discharge capacity; namely, 1161.2 mA h g−1 for SnS2 anode and 1430.5 mA h g−1 for SnS2/CNTs anode. Afterward, a rapid decline of discharge capacity occurs in the first two cycles for both electrodes, mostly due to the decomposition of electrolyte components and formation of solid-electrolyte-interface (SEI) layers on electrode surface, or the irreversible Li-ion insertion into the electrode materials (Li+ rapping effects).30 In the charge plots, a slowly increasing potential plateau is seen starting from 0.38 V to 0.7 V. It is well known that the lithiation/delithiation reactions in SnS2 have two steps and can be described by:16
| SnS2 + 4Li+ + 4e− → Sn + 2Li2S | (4) |
| Sn + xLi+ + xe− ↔ LixSn (0 ≤ x ≤ 4.4) | (5) |
In the first step, Li-ion insertion into SnS2 forms metallic Sn and amorphous Li2S. In following discharge–charge cycles, the alloy and dealloy behaviours of the newly formed Sn with Li leads to the birth and extinction of Li4.4Sn, and the Li2S acts as an inert matrix surrounding the active Sn nanograins.16 By this mechanism, Sn endows SnS2 with a theoretical capacity of 645 mA h g−1, which is rather smaller than that of pure Sn (993 mA h g−1) and SnO2 (781 mA h g−1).
Fig. 9c displays capacities of the two SnS2 electrodes over 50 electrochemical cycles at 100 mA g−1 in a potential range from 0.01 to 1.2 V versus Li/Li+. The charge capacity of SnS2 anode rises somewhat in the first five cycles, but that of SnS2/CNTs electrode does not, indicating that a Li-ion insertion activation process is negligible in the latter due to fast Li-ion transfer in a 2D nanostructure. In general, the SnS2/CNTs electrode delivers significantly better cyclability than the bare SnS2 electrode. As shown in the figure, the former shows a final charge capacity of 373.1 mA h g−1 that is 91.5% of its initial charge capacity. On the contrary, the latter exhibits a final capacity of 200.7 mA h g−1 which is only 66.8% of its initial charge capacity. The poorer cycling performance of pure SnS2 anode is attributed to its intrinsically low electronic conductivity and internal damage from large volume expansion in the alloying of Li into metallic Sn. This result proves that the strategy of composite with CNTs is as effective as carbon coatings15 or coatings on CNTs17,18 to strengthen the stability of SnS2 anodes in durable LIBs. It is worth noting that the two anodes have close capacities in the early cycling stage, which might be ascribed to the addition of superfluous MWCNTs with low capacity (<200 mA h g−1)17 into SnS2. As a result, work is in progress to optimize CNTs content in the SnS2/CNTs composite, aiming for both larger capacities and better cyclability of SnS2 anodes.
We also studied the rate performance of SnS2 and SnS2/CNTs anodes. It can be seen from Fig. 9d and e that the electrode polarization is increased faster for the SnS2 anode than for the SnS2/CNTs anode as the current goes up for tests, suggesting better conductivity of the SnS2/CNTs electrode. Consequently, the capacity of the latter becomes higher than that of pure SnS2 at various current densities from 100 to 1000 mA g−1 (Fig. 9f). In particular, the capacity of SnS2/CNTs anode is five times of that of SnS2 anode at a large current of 1000 mA g−1.
The Li-ion intercalation behaviors at the two anodes are again examined by their differential capacity curves (Fig. 10a and b) derived from their discharge–charge plots. Clearly, both of them have one broad reduction peak in the cathodic scan and one broad oxidation peak in the anodic scan. The position of the peaks is almost the same (0.05–0.39 V, 0.4–0.7 V), but the reduction peak of the SnS2/CNTs anode starts about 0.6 V and gradually rises, while that of SnS2 anode sharply increases at a lower potential of about 0.45 V, suggesting smaller polarization and better reversibility of Li-ion insertion/extraction reaction at the SnS2/CNTs anode. In addition, the estimated integral area (representing their specific capacity) between the discharge and charge plots in the whole potential range is clearly larger for the composite electrode than for the SnS2, suggesting that more electrons and Li+ ions involve the insertion/extraction process in the former. In order to verify these points, EIS technique is employed to explore Li-ion transfer kinetics at the SnS2 and SnS2/CNTs anodes. Fig. 10c shows Nyquist plots and their equivalent circuit after the cells were discharged and charged for ten cycles. A small depressed semicircle is found at high frequency region in both curves, indicating the formation of a passivating SEI film on the anode surface. A second depressed semicircle is seen in the high-to-medium frequency region, which denotes the charge transfer progress between the active material and electrolyte. In the low frequency region, the straight line displays characteristics of Warburg impedance (W) that generally reflect the Li-ion diffusion behaviours within particles of the active material. In the circuit, RΩ is the ohmic resistance derived from the organic electrolyte, separator and electrodes, corresponding to the intercept of the first depressed semicircle with Z-real axis. As for the first semicircle, Rs represents the resistance of Li-ion diffusion through the SEI layer; CPEs is the constant phase-angle element depicting the non-ideal capacitance resistance. For the second semicircle, Rct is the charge transfer resistance, and CPEdl is the constant phase-angle element that is related to a double layer capacitance. It is easily found that Rct is significantly larger for the bare SnS2 anode than for the SnS2/CNTs electrodes, which reflects rapider charge transfer or lower charge transfer resistance in the latter.
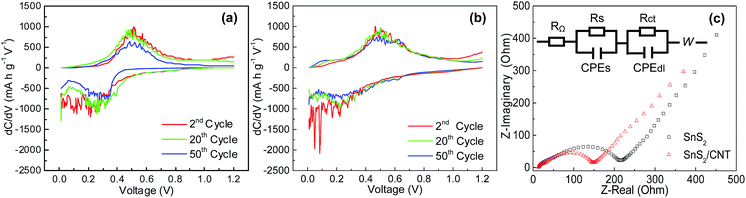 | ||
| Fig. 10 Differential capacity plots of (a) SnS2, (b) SnS2/CNTs composite, and (c) Nyquist plots and equivalent circuit. | ||
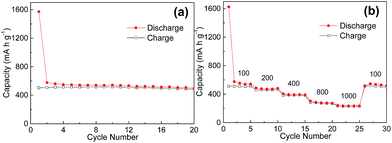
In addition to the optimization of MWCNTs content in the SnS2/CNTs composite, the oxidation of SnS2 to SnO2 could be an alternative route to improve the specific capacity of the anode, due to their theoretical capacity difference. Hence, we for the first time achieved a porous thin SnO2 nanoflake/CNTs hybrid readily by annealing the as-obtained SnS2/CNTs. Fig. 11a shows the good cycling performance of SnO2/CNTs anode at 100 mA g−1 over 20 cycles. Apparently, its capacity of about 500 mA h g−1 exceeds that of SnS2/CNTs anode by almost 100 mA h g−1, let alone graphite with an even lower capacity. The SnO2/CNTs electrode also delivers excellent rate capability at various current from 100 to 1000 mA g−1 (Fig. 11b). The results demonstrate that a hybrid structure made of 2D active material (e.g. metal oxides and sulfides) and conductive carbon network is an ideal candidate for high-performance LIB electrodes.
3. Conclusions
SnS2 nanoparticles and SnS2 nanoflake/CNTs composite were prepared by a simple hydrothermal approach. The addition of conducting MWCNTs for the synthesis was found to affect the morphology of obtained SnS2 nanostructures. Dispersed CNTs in solutions restrain their growth and yield the formation of 2D SnS2 nanoflakes enwrapped by MWCNTs, rather than irregular SnS2 nanoparticles, eventually to achieve a 3D porous structure with larger surface area. Firstly, those nanoflakes shorten paths for Li-ion transfer and thus reduce the ionic transfer resistance. Secondly, the embedment of SnS2 nanoflakes into a conductive MWCNT matrix allows fast electronic diffusion and effectively cushions the volume change of SnS2 during cycling. All these unique properties lead to the significantly better cyclability and rate performance of SnS2/CNTs composite than that of the SnS2 nanoparticle anode. The composite can be fully changed to 2D SnO2 nanoflake/CNTs hybrid via annealing in air, which shows increased Li-ion storage capacities. Our results demonstrate that metal oxides and sulphides that are integrated with conducing MWCNTs and therefore harvest enhanced Li-ion intercalation properties could be a promising material for the anode of high-performance LIBs.4. Experimental details
MWCNTs (outer diameter of 6–9 nm, length of 5 μm, Sigma Aldrich) were first added into nitric acid and stirred overnight, then filtered and washed with de-ionized water and absolute ethanol (≥99.5%, Sigma Aldrich), and dried in a vacuum oven. 0.009 g CNTs and 1 g sodium dodecylbenzenesulfonate (SDBS) powder were put into a glass beaker containing 60 ml anhydrous ethanol, ultrasonically treated until a homogenous black solution was obtained. Later, 0.001 mol SnCl2·2H2O and 0.004 mol thiourea powder were added into the CNTs solution. After vigorously stirring for 15 min, the solution was transferred into a Teflon-lined autoclave of 100 ml capacity, and then the autoclave was sealed and heated at 200 °C for 24 h. A black precipitate was yielded and collected by centrifugation, rinsed with distilled water and ethanol for six times. The final product was dried at 80 °C in the vacuum oven overnight. For comparison, SnS2 particles were produced in the same route but without the addition of CNTs. The obtained SnS2/CNTs product was further annealed at 500 °C in air for 1 h to gain SnO2/CNTs composites.A Hitachi S-4800 scanning electron microscope (SEM) and a Hitachi H9000NAR transmission electron microscope (TEM) were used to observe these materials. Their crystallographic structure was determined by X-ray diffraction (XRD) using a Scintag XDS 2000 X-ray diffractometer with Cu Kα radiation at a scan rate of 2° min−1. Chemical composition of these particles was characterized by an energy dispersive X-ray spectroscopy (EDS) with an XFlash detector (Bruker AXS), and an X-ray photoelectron spectroscope (XPS, Perkin-Elmer PHI 5400 ESCA System) using a monochromatized Mg Kα X-ray radiation source. Thermogravimetry analysis (TGA) was carried out on a simultaneous thermal analyzer (TA Instruments, SDT 2960) in an air atmosphere from room temperature to 1000 °C at a rate of 20 °C min−1. The Brunauer–Emmett–Teller (BET) surface area were measured by a surface area and porosity analyzer (MICROMERITICS, ASAP 2020).
Cu foils (12 μm thick) were cleaned with acetone and dried in air. A slurry was made from a mixture of the prepared material, carbon black (99.5%, Alfa Aesar), and polyvinylidene fluoride (4 wt% in N-methyl-2-pyrrolidone) in a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The slurry was then spread on the copper foil using a blade casting method, followed by drying in air at room temperature and in vacuum at 100 °C for 12 h. Later, the Cu foil was cut into disks with a diameter of 3/8 inch that were then pressed by a Carver Lab Press with a pressure of 6 MPa. Masses of the active material are about 1 mg. CR2032 coin cells were assembled to investigate battery behaviours of the samples acting as the working electrode. A lithium disc is used as the counter and reference electrode in the cells, which was separated from the working electrode by a Celgard-2320 separator. The organic electrolyte was 1 mol L−1 LiPF6 dissolved in a mixed solvent of ethylene carbonate and diethylene carbonate with 1
10. The slurry was then spread on the copper foil using a blade casting method, followed by drying in air at room temperature and in vacuum at 100 °C for 12 h. Later, the Cu foil was cut into disks with a diameter of 3/8 inch that were then pressed by a Carver Lab Press with a pressure of 6 MPa. Masses of the active material are about 1 mg. CR2032 coin cells were assembled to investigate battery behaviours of the samples acting as the working electrode. A lithium disc is used as the counter and reference electrode in the cells, which was separated from the working electrode by a Celgard-2320 separator. The organic electrolyte was 1 mol L−1 LiPF6 dissolved in a mixed solvent of ethylene carbonate and diethylene carbonate with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio (Novolyte Technologies, Inc.). All the coin cells were assembled in an Ar-filled glove box with oxygen and moisture levels lower than 1 ppm. The galvanostatic charge–discharge was carried out on LANHE automatic battery testers at 100 mA g−1 current for 50 cycles or 100–1000 mA g−1 for 30 cycles. AC impedance measurements were also conducted on these cells at a potential of ∼0.4 V from 100 kHz to 0.01 Hz with a perturbation of 5 mV applied.
1 volume ratio (Novolyte Technologies, Inc.). All the coin cells were assembled in an Ar-filled glove box with oxygen and moisture levels lower than 1 ppm. The galvanostatic charge–discharge was carried out on LANHE automatic battery testers at 100 mA g−1 current for 50 cycles or 100–1000 mA g−1 for 30 cycles. AC impedance measurements were also conducted on these cells at a potential of ∼0.4 V from 100 kHz to 0.01 Hz with a perturbation of 5 mV applied.
Acknowledgements
Financial supports from the University of Wisconsin System Applied Research program and the University of Wisconsin–Milwaukee Bradley and Hertz Catalyst Grant Programs are gratefully acknowledged.Notes and references
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef CAS PubMed.
- J. Li, Y. Hou, X. Gao, D. Guan, Y. Xie, J. Chen and C. Yuan, Nano Energy, 2015, 16, 10 CrossRef PubMed.
- Q. Wang, Q. Wang, D. A. Zhang, J. Sun, L. L. Xin and X. Y. Xue, Chem.–Asian J., 2014, 9, 3299 CrossRef CAS PubMed.
- Q. Wang, D. A. Zhang, Q. Wang, J. Sun, L. L. Xin and X. Y. Xue, Electrochim. Acta, 2014, 146, 411 CrossRef CAS PubMed.
- Q. Wang, J. Sun, Q. Wang, D. A. Zhang, L. L. Xin and X. Y. Xue, J. Mater. Chem. A, 2015, 3, 5083 CAS.
- M. K. Jana, H. B. Rajendra, A. J. Bhattacharyya and K. Biswas, CrystEngComm, 2014, 16, 3994 RSC.
- S. H. Chaki, M. P. Deshpande, D. P. Trivedi, J. P. Tailor, M. D. Chaudhary and K. Mahato, Appl. Nanosci., 2013, 3, 189 CrossRef CAS.
- J. Seo, J. Jang, S. Park, C. Kim, B. Park and J. Cheon, Adv. Mater., 2008, 20, 4269 CrossRef CAS PubMed.
- T. J. Kim, C. Kim, D. Son, M. Choi and B. Park, J. Power Sources, 2007, 167, 529 CrossRef CAS PubMed.
- D. Ma, W. Zhang, Q. Tang, R. Zhang, W. Yu and Y. Qian, J. Nanosci. Nanotechnol., 2005, 5, 806 CrossRef CAS PubMed.
- S. Liu, X. Yin, L. Chen, Q. Li and T. Wang, Solid State Sci., 2010, 12, 712 CrossRef CAS PubMed.
- P. Wu, N. Du, H. Zhang, J. Liu, L. Chang, L. Wang, D. Yang and J. Z. Jiang, Nanoscale, 2012, 4, 4002 RSC.
- K. Chang, Z. Wang, G. Huang, H. Li, W. Chen and J. Y. Lee, J. Power Sources, 2012, 201, 259 CrossRef CAS PubMed.
- H. S. Kim, Y. H. Chung, S. H. Kang and Y. E. Sung, Electrochim. Acta, 2009, 54, 3606 CrossRef CAS PubMed.
- M. He, L. X. Yuan and Y. H. Huang, RSC Adv., 2013, 3, 3374 RSC.
- C. Zhai, N. Du, H. Zhang, J. Yu and D. Yang, ACS Appl. Mater. Interfaces, 2011, 3, 4067 CAS.
- H. Sun, M. Ahmad, J. Luo, Y. Shi, W. Shen and J. Zhu, Mater. Res. Bull., 2014, 49, 319 CrossRef CAS PubMed.
- J. G. Kang, G. H. Lee, K. S. Park, S. O. Kim, S. Lee, D. W. Kim and J. G. Park, J. Mater. Chem., 2012, 22, 9330 RSC.
- D. A. Zhang, Q. Wang, Q. Wang, J. Sun, L. L. Xing and X. Y. Xue, Mater. Lett., 2014, 128, 295 CrossRef CAS PubMed.
- M. O. Guler, O. Cevher, T. Cetinkaya, U. Tocoglu and H. Akbulut, Int. J. Energy Res., 2014, 38, 487 CrossRef CAS PubMed.
- D. Su, H.-J. Ahnb and G. Wang, Chem. Commun., 2013, 49, 3131 RSC.
- L. Zhu, W. Wu, Y. Zhu, W. Tang and Y. Wu, J. Phys. Chem. C, 2015, 119, 7069 CAS.
- X. Zhou, Z. Xia, Z. Zhang, Y. Ma and Y. Qu, J. Mater. Chem. A, 2014, 2, 11799 CAS.
- J. Huang, K. Yu, C. Gu, M. Zhai, Y. Wu, M. Yang and J. Liu, Sens. Actuators, B, 2010, 147, 467 CrossRef CAS PubMed.
- Y. Z. Zhang, H. Pang, Y. Sun, W. Y. Lai, A. Wei and W. Huang, Int. J. Electrochem. Sci., 2013, 8, 3371 CAS.
- J. Liu, J. Liu, W. Song, F. Wang and Y. Song, J. Mater. Chem., 2014, 2, 17477 RSC.
- A. M. Baker, L. Wang, S. G. Advani and A. K. Prasad, J. Mater. Chem., 2012, 22, 14008 RSC.
- H. Zhong, G. Yang, H. Song, Q. Liao, H. Cui, P. Shen and C. X. Wang, J. Phys. Chem. C, 2012, 116, 9319 CAS.
- D. Guan, J. Li, X. Gao and C. Yuan, J. Alloys Compd., 2014, 617, 464 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: SEM photos, BET curves, EDS spectrum, XPS spectra. See DOI: 10.1039/c5ra09613h |
| This journal is © The Royal Society of Chemistry 2015 |