One-pot synthesis of Ag-modified LaMnO3–graphene hybrid photocatalysts and application in the photocatalytic discoloration of an azo-dye
Jie
Hu
ab,
Jie
Men
b,
Yuanyuan
Liu
b,
Hao
Huang
*a and
Tifeng
Jiao
a
aState Key Laboratory of Metastable Materials Science & Technology, Yanshan University, Qinhuangdao, 066004, P.R. China. E-mail: huanghao@ysu.edu.cn; Fax: +86-335-8074545; Tel: +86-15369700375
bHebei Key Laboratory of Applied Chemistry, Department of Environment and Chemistry, Yanshan University, Qinhuangdao, 066004, P.R. China
First published on 9th June 2015
Abstract
Ag-modified LaMnO3–graphene nanocomposites were successfully synthesized by the sol–gel technique. The nanocomposites were characterized by X-ray diffraction, field-emission scanning electron microscopy with energy dispersive spectrometer, transmission electron microscopy, X-ray photoelectron spectroscopy, Brunauer–Emmett–Teller surface area analysis and photoluminescence analysis. High-efficiency degradation of Direct Green BE under UV-vis light was achieved for Ag/LaMnO3–graphene as photocatalyst. The enhancement of UV-vis photocatalytic activity can be attributed to the high separation efficiency of photoinduced electron–hole pairs resulting from the excellent conductivity of Ag in composites and the formation of multistage drill way, which can promote the adsorption of organic dyes and improve the transfer efficiency of the photocatalytic process. The photocatalytic mechanism was studied by adding hydroxyl radical (˙OH), superoxide radical (˙O2−), and active hole (h+) scavengers. The results confirmed that h+ generated in Ag/LaMnO3–graphene played a key role in photocatalysis, with the assistance of ˙OH. By contrast, ˙O2− had the least influence on the process.
1. Introduction
Organic dyes are commonly present in the effluent of various industries, such as in the production of textiles, leather goods, plastics, cosmetics, consumer electronics, and food.1–4 Modern commercial dyes are characterized by their strong structural and color stability, which is imparted by their high degree of aromaticity and extensively conjugated chromophores.5 Among the various commercial synthetic dyes, azo dyes comprise the largest class with a wide variety of colors and structures; this group represents up to 70% of the total textile dyestuffs used.6 To date, photocatalytic technologies are widely applied because of their potential capability to completely degrade azo dye molecules into mineralized end-products, such as CO2 and H2O.7TiO2 is a well-studied semiconductor photocatalyst because of its low cost, strong oxidizing power, and nontoxic nature; the effectiveness of this photocatalyst for degrading pollutants has been well documented.8 However, TiO2 can only be activated by UV irradiation (λ < 400 nm) because of its relatively wide band gap (3.2 eV), which comprises less than 5% of the solar spectrum.9 To maximize the use of sunlight, photocatalysts with the perovskite (ABO3) structure have been recently exploited as highly effective photocatalysts.10 However, electron (e−)–hole (h+) recombination easily occurs during photocatalytic degradation by pure perovskite. Several researchers have employed different strategies to solve this problem, such as the partial substitution11 and nitrogen doping of A or B ions,12 coupling with semiconductors,13 or modification with noble metals.14
More recently, silver has become an emerging photocatalyst of great interest to researchers worldwide.15 Du et al.16 showed that Ag/TiO2 core–shell nanowires with absorption in the visible region may present higher activity during photocatalysis. Liu17 and Lu et al.18 reported that Ag nanoparticles have a high capacity for electron capture, which promotes the higher separation efficiency of e−–h+ and largely enhances the photocatalytic degradation under irradiation with UV-visible light. In addition, Lee et al.19 proposed that Ag worked as electron capture agent on the surface of CaTiO3 to reduce the e−–h+ recombination.
Graphene is a two-dimensional (2-D) monolayer of sp2 hybridized carbon atoms with an unprecedented large specific surface area, flexible structure, excellent mobility of charge carriers, and good electrical conductivity.20–23 Numerous studies24–26 have shown that the introduction of graphene enhanced the harvesting efficiency of light, reduce the recombination rate of e−–h+, increased the adsorption of dye, and consequently improved the photocatalytic activity.
Research on the preparation of 3D composite photocatalysts has rapidly developed recent years. The synergistic effect of its three components makes the performance of a 3D composite photocatalyst much better than that of the pure photocatalyst or the 2D composite photocatalyst.27–29 In this work, Ag/LaMnO3–graphene nanocomposites were synthesized successfully via the sol–gel technique, and their photocatalytic performance was examined through the degradation of Direct Green BE. The photocatalytic mechanism was also investigated by scavengers of hydroxyl radicals (˙OH), superoxide radicals (˙O2−), and active h+via the photoluminescence (PL) technique. Additionally, the synergistic effect of Ag, LaMnO3, and graphene on the photocatalytic activity was systematically elucidated. To investigate the reusable stability of the as-synthesized catalysts, cycling runs of the composite under UV-vis light irradiation were conducted.
2. Experimental section
2.1. Sample synthesis
Thin-layer graphene materials were synthesized from natural graphite (Qingdao Graphite Company) using a modified Hummer preparation.30 All the raw materials were analytical grade and used without further purification. Samples of the Ag/LaMnO3–graphene composite with varied Ag content were prepared from La(NO3)3, Mn(NO3)2, and AgNO3 by the sol–gel method.In a typical experiment, sol–gel synthesis can be described as follows: A stoichiometric ratio of 0.1 mol L−1 solutions of La(NO3)3 and Mn(NO3)2 were mixed with a certain amount of the 0.1 mol L−1 AgNO3 solution (the quality ratio of Ag to LaMnO3 was varied at 2–6%). Alkylphenol polyoxyethylene (OP-10) was used as the dispersing agent (at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 with respect to the cations), and citric acid was the chelating agent (at a molar ratio of 2
9 with respect to the cations), and citric acid was the chelating agent (at a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with respect to the cations). Both were added to the mixture, dispersed with magnetic stirring, and mixed with 100 mL of the graphene water suspension (0.1 g L−1), which was followed by ultrasonic dispersion for 1 h. The obtained suspension was adjusted to pH of ∼9 with aqueous ammonia. The suspension was aged in a water-bath of ∼60 °C until the xerogel had formed. The xerogel was burned at 350 °C for 5 h in air, then the burnt remains were calcinated at 650 °C for 4 h in a vacuum furnace to form the desired samples of x wt% Ag/LaMnO3–graphene (where x is the quality ratio). For comparison, the pure LaMnO3 powders and LaMnO3–graphene composites were prepared under the same conditions.
1 with respect to the cations). Both were added to the mixture, dispersed with magnetic stirring, and mixed with 100 mL of the graphene water suspension (0.1 g L−1), which was followed by ultrasonic dispersion for 1 h. The obtained suspension was adjusted to pH of ∼9 with aqueous ammonia. The suspension was aged in a water-bath of ∼60 °C until the xerogel had formed. The xerogel was burned at 350 °C for 5 h in air, then the burnt remains were calcinated at 650 °C for 4 h in a vacuum furnace to form the desired samples of x wt% Ag/LaMnO3–graphene (where x is the quality ratio). For comparison, the pure LaMnO3 powders and LaMnO3–graphene composites were prepared under the same conditions.
2.2. Sample characterization
Phase identification of the as-synthesized samples were performed by X-ray diffraction (XRD D/max-2500/pc) using Cu Kα (λ = 0.15405 nm) radiation with scanning angles of 10–90°. Morphology of the composites was investigated by transmission electron microscopy (TEM JEOL-2010) with an accelerating voltage of 200 kV and field-emission scanning electron microscopy (FE-SEM Hitachi S-4800) coupled with energy-dispersive spectrometry (EDS). Binding energy of the elements was measured at about 20 °C by X-ray photoelectron spectroscopy (XPS ESCALAB 250) with a monochromatized Al–Mg X-ray source (Al hν = 1486.6 eV; Mg hν = 1253.6 eV). Brunauer–Emmett–Teller (BET) specific surface areas were evaluated based on nitrogen adsorption isotherms at −196 °C on a NOVE 4000e analyzer. The absorption spectra of samples were recorded with a Shimadzu UV-2550 spectrophotometer in the range of 200 nm to 800 nm. Photoluminescence (PL) spectra were obtained at room temperature on a Fluorescence Spectrophotometer (F-7000, Hitachi, Japan) with a Xenon lamp at an excitation wavelength of 400 nm. Scanning speed was 1200 nm min−1, and PMT voltage was 700 V. The width of excitation and emission slits were both 10.0 nm.2.3. Photocatalytic activity test
The photocatalytic activity was determined by the degradation of Direct Green BE in water under the irradiation of a xenon lamp (CHF-XM-300 W; Beijing Trusttech Co., Ltd., China). To measure the photocatalysis, 0.02 g of the respective photocatalyst was added to 50 mL of Direct Green BE aqueous solution. The initial dye concentration was 0.02 g L−1, which is defined as C0. Before illumination, the suspension was magnetically stirred in the dark for 30 min to attain a physical adsorption–desorption equilibrium. After every 30 min, 4 mL of the suspension was continuously sampled; each sample was centrifuged at 4000 rpm for 5 min to remove the solid particles. The obtained clear solution in the upper layer was analyzed at a range of 200–800 nm by UV-2550 spectrophotometer (Shimadzu UV-2550). The total organic carbon (TOC) at different irradiation times was determined with a Shimadzu TOC Analyzer (Shimadzu TOC-VCPH). The degradation percentage of the dye is defined as C/C′0, where C′0 and C are the dye concentrations after adsorption–desorption equilibrium and irradiation, respectively.2.4. Measurement of hydroxyl radicals
At room temperature, 0.1 g of 2 wt% Ag/LaMnO3–graphene was dispersed in 20 mL of 10−3 M coumarin (COU Alfa Aesar Co., Ltd) aqueous solution. Before illumination, the solution was magnetically stirred in darkness for 30 min to reach adsorption–desorption equilibrium among the photocatalyst, COU, and water. To produce 7-hydroxycoumarin (7HC) from ˙OH radicals and COU, the solution was continuously stirred under irradiation. After every 30 min, the reaction solution was filtered to measure PL intensity. The equation is shown below:31| COU + ˙OH → 7HC |
PL spectra of generated 7HC were obtained at an excitation wavelength of 335 nm. Scanning speed was 1200 nm min−1, and PMT voltage was 700 V. Width of excitation and emission slit were both set to 5.0 nm.
3. Results and discussion
3.1. Catalyst characterization
XRD is an effective method to investigate the solid structure of nanomaterials and to confirm their phase purity. Compared with graphite, the graphene sheet has a low-intensity diffraction peak for C (002) at 2θ = 21.5° (Fig. 1), which indicates the short-range order of the graphene sheets. The XRD data of LaMnO3 can be indexed to a perovskite-type structure, without any miscellaneous peaks, which coincides well with the reported data (PDF #50-0297). Given the low graphene content in the composites and the disordered stacking of the graphene sheets, the primary diffraction peaks of the LaMnO3–graphene composites are similar to that of LaMnO3. Therefore, the presence of graphene did not change the perovskite-type structure. The obtained diffraction peaks (2θ = 38.116°, 44.277°, 64.426°, 77.472°, 81.536°) were intense and sharp in the Ag/LaMnO3–graphene samples with different Ag content; these peaks correspond to the (111), (200), (220), (311), and (222) planes, respectively, of the face-centered cubic (fcc) structure of Ag (PDF #04-0783).32 These results indicated that the Ag nanoparticles were well crystallized; no other phases were observed, not even those for AgxO. Therefore, Ag is highly chemically compatible with perovskite-type materials in its metal form; the peak intensities of metallic silver were slightly increased with an increasing AgNO3 concentration. The average crystallite sizes of LaMnO3 and Ag microcrystal in 2 wt% Ag/LaMnO3–graphene were approximately 22 and 19 nm, respectively, which was calculated in terms of the Scherrer’s formula based on the diffraction peak of LaMnO3 (110) and Ag (111). | ||
| Fig. 1 XRD patterns of graphene, LaMnO3, LaMnO3–graphene and different contents of Ag on Ag/LaMnO3–graphene and standard patterns of LaMnO3 and Ag. | ||
An atomic force microscopy (AFM) morphological image and the corresponding cross-section analysis of graphene on the silicon substrate are presented in Fig. 2a. The average thickness of sample was approximately 1 nm in Fig. 2a-2, thereby indicating that a thin graphene sheet was obtained. As shown in Fig. 2b, the as-prepared graphene structure has gauze-shaped wrinkles and folds, which may be caused by oxygenic functional groups, such as carboxyl and carbonyl group, and other resultant defects during the preparation of graphene oxide. FE-SEM was performed to observe the morphology of the as-synthesized material. A typical FE-SEM image of the 2 wt% Ag/LaMnO3–graphene composite exhibited a porous structure (Fig. 2c). As a photocatalyst, the porous structure could provide abundant channels for the spread of the dye, promote dye adsorption, and greatly increase the chance of reaction between the catalyst and dye, thereby improving the photocatalytic properties. The as-prepared graphene was decorated with dense perovskite and metallic Ag nanoparticles (Fig. 2c and d), which decreased the agglomeration of nanoparticles. EDS data of the 2 wt% Ag/LaMnO3–graphene composite (Fig. 2e) indicated the presence of the elements C, O, Mn, Ag, and La. The quality ratio of Ag to LaMnO3 was approximately 1.9%, which agreed with the added amount. The C signal was much weaker than the intensity of La and Mn signals, which may be attributed to the low graphene content. The O content was higher than the theoretical value because of the residual oxygenic functional groups in graphene. The TEM image is shown in Fig. 2f. LaMnO3 and Ag had interplanar distances of approximately 0.386 and 0.236 nm, which corresponded to the (110) and (111) planes, respectively. The space lattice of graphene was not obvious in the TEM images because graphene is thin and transparent. This result indicates that no changes occurred in the lattice structure of LaMnO3 and Ag after graphene was introduced, and the nanoparticles exhibited a highly crystalline structure.
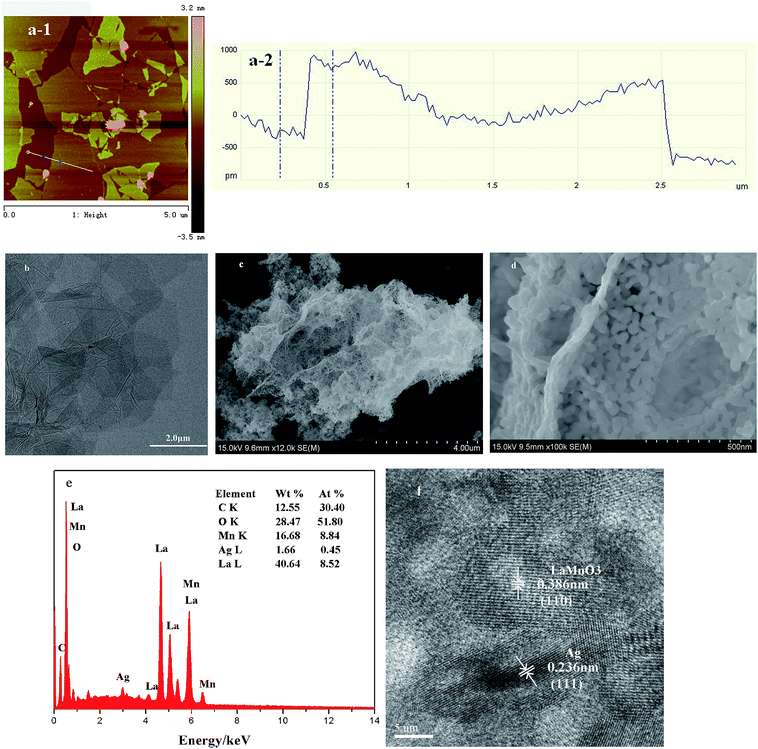 | ||
| Fig. 2 (a) AFM image of as-synthesized graphene (b) TEM image of graphene (c–f) SEM, EDS and HRTEM images of 2 wt% Ag/LaMnO3–graphene composite. | ||
 | ||
| Fig. 3 XPS spectra of 2 wt% Ag/LaMnO3–graphene composite (a) overall spectrum and high-resolution curves of (b) Ag 3d, (c) Mn 2p and (d) C 1s region. | ||
XPS data (Fig. 3a) showed that the as-prepared sample was composed of C, Ag, O, Mn, and La elements, thereby verifying that the Ag elements remained on the nanocomposite surface. As shown in Fig. 3b, the Ag 3d5/2 peak was found at 368.4 eV and the Ag 3d3/2 peak appeared at 374.4 eV,16 whereas the splitting of the 3d doublet was 6 eV, thereby indicating that Ag exists in its metal state in the sample.17 The Mn 2p spectra in Fig. 3c shows that the main peaks were located at 641.5 and 653 eV, which represented Mn 2p3/2 and Mn 2p1/2, respectively. The range of Mn 2p3/2 and Mn 2p1/2 could be divided into two peaks by fitting, respectively. The peaks at 653.9 and 642.4 eV were assigned to Mn4+, while the peaks at 653.4 and 641.1 eV were assigned to Mn3+. The core-level XPS spectra of C 1s and the corresponding deconvoluted spectra for graphene are shown in Fig. 3d, with two strong peaks centered at approximately 284.3 and 288.5 eV. The peak located at 283.4 eV is a characteristic of graphitic carbon; this peak corresponds to the C–C bond. The deconvoluted peaks with binding energies of 284.6, 287.3, and 289 eV imply the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O, and O–C
C, C–O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively. The XPS peak area ratios of the C
O bonds, respectively. The XPS peak area ratios of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond to the C–C bond showed that most of the carbon atoms were sp2 hybridized, thereby implying that most of the oxygen-containing functional groups of graphene in Ag/LaMnO3–graphene were removed.20
O bond to the C–C bond showed that most of the carbon atoms were sp2 hybridized, thereby implying that most of the oxygen-containing functional groups of graphene in Ag/LaMnO3–graphene were removed.20
The microstructural characteristics of 2 wt% Ag/LaMnO3–graphene were further investigated with the N2 adsorption–desorption isotherms, which are shown in Fig. 4a. The characteristics of type IV isotherms were demonstrated, and a significant hysteresis loop at high relative pressure could be seen (P/P0 of 0.75–0.95), thereby indicating that the samples were mesoporous materials. The pore size distribution curves were calculated by BJH methods, and the properties of the samples are summarized in Table 1. The pore size for pure LaMnO3 ranged from 3–4 nm, while that of 2 wt% Ag/LaMnO3–graphene was 3–8 nm (Fig. 4b). The hierarchically porous structure of 2 wt% Ag/LaMnO3–graphene can adsorb more nitrogen and has a relatively high BET specific surface area of 39.709 m2 g−1, which is larger than that of pure LaMnO3 (29.981 m2 g−1). Furthermore, the pore volume and average pore diameter were also larger than that of pure LaMnO3. Higher specific surface areas can increase the number of catalyst activity points as well as the chance of contact between pollutant molecules and the active point. Larger pore diameters and pore volumes can offer abundant channels for the dye solution and reduce the mass transfer resistance of the reaction process, thereby making it easier for the pollutant molecules to reach the active points, which improves the photocatalytic performance.
 | ||
| Fig. 4 N2 adsorption–desorption isotherms (a) and pore size distribution (b) of 2 wt% Ag/LaMnO3–graphene and pure LaMnO3. | ||
| Sample | Specific surface area (m2 g−1) | Average pore diameter (nm) | Pore volume (cm3 g−1) |
|---|---|---|---|
| 2 wt% Ag/LaMnO3–graphene | 39.709 | 12.045 | 0.218 |
| Pure LaMnO3 | 29.981 | 11.663 | 0.128 |
3.2. UV-vis light photocatalytic activity
The photocatalytic activity of the Ag/LaMnO3–graphene nanocomposites was evaluated by using the photocatalytic degradation of the azo dye Direct Green BE as a model reaction. For comparison, the catalytic properties of LaMnO3 and LaMnO3–graphene were also studied. It can be seen from Fig. 5a that the adsorption of dye under different samples without illumination all attain adsorption–desorption equilibrium at 30 min. Because of the hierarchically porous structure of Ag/LaMnO3–graphene, the samples have larger adsorption for dyes. Compared with LaMnO3 and LaMnO3–graphene, after lighting for 3 h by using Ag/LaMnO3–graphene as photocatalysts, the absorption peak intensity of the dye weakened dramatically (Fig. 5b). When the quality ratio of Ag to LaMnO3 was varied at 2%, the main absorption peaks of Direct Green were almost completely eliminated. Plots of the degradation ratio after lighting versus reaction time (Fig. 5c) in the presence of different photocatalysts were obtained. Ag/LaMnO3–graphene showed much better photocatalytic performance. Among the composite catalysts, 2 wt% Ag/LaMnO3–graphene had the best performance, whose decolorization ratio reached 98% within 3 h. The degradation process clearly complies with the reaction for pseudo-first-order kinetics: ln(C/C′0) = −kt (Fig. 5d), where k is the degradation constant. It was 0.8164 h−1 for 2 wt% Ag/LaMnO3–graphene (Table 2), which was much larger than that of the others catalysts in this study. Fig. 5e shows the UV-vis DRS spectra of LaMnO3, LaMnO3–graphene and 2 wt% Ag/LaMnO3–graphene. As shown in the figure, the light absorption of 2 wt% Ag/LaMnO3–graphene in the UV and visible are both significantly increased compared with that of LaMnO3 and LaMnO3–graphene, which indicates that the modification of Ag expands the light response range. These results clearly explain that the combination of Ag and LaMnO3–graphene can greatly raise the photocatalytic activity of the catalysts.| Catalyst | R 2 | k/h−1 |
|---|---|---|
| LaMnO3 | 0.9765 | 0.2689 |
| LaMnO3–graphene | 0.9915 | 0.3894 |
| 2 wt% Ag/LaMnO3–graphene | 0.9908 | 0.8164 |
| 4 wt% Ag/LaMnO3–graphene | 0.9891 | 0.6433 |
| 6 wt% Ag/LaMnO3–graphene | 0.9872 | 0.5393 |
PL spectra have been widely used to investigate the transfer behavior of photogenerated e− and h+ in solid semiconductor materials such that they can be used to interpret the separation efficiency of these e− and h+.17,33 The PL spectra of samples with an excitation wavelength of 400 nm are shown in Fig. 5f. Two broad and gentle emission bands were observed in the scanning range of 440–480 nm. A broad peak appeared at approximately 440–460 nm and originated from the free exciton emission; a second emission band peaked at approximately 470 nm and was ascribed to the bound exciton emission.34 The intensity of the PL spectra of Ag/LaMnO3–graphene composites was much weaker than those of LaMnO3 and LaMnO3–graphene; and among these composites, the 2 wt% Ag/LaMnO3–graphene catalyst had the weakest spectra. This result indicates that an appropriate amount of Ag (2 wt%) doping can slow the recombination rate of photogenerated e− and h+ in LaMnO3 and improve the photocatalytic activity.
UV-visible spectra of the degradation of Direct Green BE by 2 wt% Ag/LaMnO3–graphene was shown in Fig. 5g. The degradation ratio (1.6% degradation) without catalyst is relatively low, which suggests that the photodecomposition can be neglected. After stirring in the dark for 0.5 h, 43% of the dye was adsorbed. The characteristic absorption peak at 376 and 617 nm decreased quickly with increasing irradiation time, which indicates that the 2 wt% Ag/LaMnO3–graphene nanocomposites successfully catalyzed the degradation of Direct Green BE. After irradiation for 3 h, the intense green color of the initial dye solution had faded (insert in Fig. 5g). The insert in Fig. 5g shows that the TOC decreased with increasing irradiation time and reached 0.654 mg L−1 after irradiation for 3 h. To ensure that the dye was completely degraded, the irradiation time was increased. When the irradiation time was increased to 5 h, the TOC was 0.285, which is almost similar to that of water (0.272 mg L−1).
3.3. Identification of reactive oxygen radicals
During photodegradation, ˙OH, ˙O2−, and h+ are the well-known dominant active species.35 Therefore, the photocatalytic reaction mechanism of the pollutant was investigated through trapping experiments of the main active species by the presence of scavengers during the photocatalytic process. In the present study, a series of experiments were conducted in the presence of 2 wt% Ag/LaMnO3–graphene by adding isopropyl alcohol (IPA)36 as an ˙OH scavenger and ethylenediaminetetraacetic acid (EDTA)37,38 as an effective h+ scavenger. Nitrogen (N2)37 was added to evaluate the effect of dissolved oxygen. A blank experiment was performed without scavengers under identical conditions. As shown in Fig. 6a, the degradation efficiency was slightly reduced with the addition of IPA (˙OH scavenger) and N2 (˙O2− scavenger), which indicated that the photodegradation was slightly inhibited under anoxic conditions. The above-mentioned experiments showed that almost no dye degradation occurred when EDTA was added to the reaction solution. Relatively speaking, these results reveal that the h+ has the most important role during photodegradation, whereas ˙OH has a secondary role; ˙O2− radicals have the least influence on the degradation.To further ascertain the generation of ˙OH during degradation under UV-vis light irradiation, the PL technique was employed with COU as a probe molecule. COU can easily react with ˙OH to produce the highly fluorescent product 7HC31 (inset in Fig. 6b). The generated PL spectra had an identical shape at approximately 461 nm, but a slight change in the PL intensity was observed with increasing irradiation time, as shown in Fig. 6b. This result suggested that the fluorescent product 7HC was formed by the specific reaction between ˙OH and COU during photocatalysis. Therefore, a small amount of ˙OH was probably produced at the 2 wt% Ag/LaMnO3–graphene surface, but no obvious changes were observed with increasing irradiation time. The above-mentioned results further indicated that h+ radicals are mainly responsible for the degradation of Direct Green BE in the presence of 2 wt% Ag/LaMnO3–graphene.
3.4. Mechanism for the enhanced photocatalytic activity
A possible mechanism for photodegradation was proposed based on the abovementioned investigation (Fig. 7). When the prepared photocatalyst is irradiated with UV-vis light, photogenerated e− are produced in the conduction band (CB) and photogenerated h+ generate in the valence band (VB),23 as shown in eqn (1). e− in CBLaMnO3–graphene can transfer to this level and react with oxygen to produce ˙O2− species32viaeqn (2). Moreover, h+ has opportunity to react with OH−/H2O to form ˙OH radicals through eqn (3). Subsequently, ˙O2−, h+, and ˙OH directly oxidize organic molecules, such as Direct Green BE viaeqn (4) to (6). In LaMnO3–graphene composites, e− could be transported along the graphene sheets from the inner region to reduce the recombination ratio of the e−–h+ pairs. Furthermore, because a Schottky barrier is formed between metallic Ag and LaMnO3, Ag is an electron capture agent that can capture e− that have been photogenerated or transported along the graphene sheets, thereby hindering the recombination of the e−–h+ pairs and leading to effective charge separation and stabilization. The synergistic effects of Ag and graphene improve the photodegradation of the system.| Ag/LaMnO3–graphene + hν → e− + h+ | (1) |
| O2 + e− → ˙O2− | (2) |
| h+ + OH− → ˙OH | (3) |
| Dye + ˙O2− → Products | (4) |
| Dye + h+ → Products | (5) |
| Dye + ˙OH → Products | (6) |
 | ||
| Fig. 7 Proposed mechanism for the photocatalytic degradation of Direct Green BE over Ag/LaMnO3–graphene nanocomposites under visible-light irradiation. | ||
3.5. Stability and reusability
The practical treatment of wastewater requires photocatalysts that are stable and reusable. Therefore, the stability and reusability of 2 wt% Ag/LaMnO3–graphene were investigated. After the Direct Green BE solution was decolorized by the photocatalytic operation, the reused 2 wt% Ag/LaMnO3–graphene was recycled after filtration, washed with deionized water, and dried at 100 °C for 8 h. The recycled 2 wt% Ag/LaMnO3–graphene was used for the second cycle of Direct Green BE degradation. As shown in Fig. 8, no significant changes in the photocatalytic activity were observed during repeated use for ten cycles, and the decolorization ratio still reached 97.8%, thereby indicating that the catalyst is stable and possesses favorable recycling characteristics.4. Conclusions
Ag-modified LaMnO3–graphene nanocomposites were successfully synthesized via the sol–gel technique. The photocatalytic ability was evaluated by the degradation of Direct Green BE. The photocatalytic efficiency was higher in Ag-modified LaMnO3–graphene than in pure LaMnO3 and LaMnO3–graphene because of the synergistic effects between Ag and graphene. The photoreaction followed the pseudo-first-order kinetic model, and the holes were the main characteristics. This investigation may lead to new opportunities for the simulated sunlight-induced photocatalysis by Ag/LaMnO3–graphene and its practical application in the photodegradation of organic pollutants.Acknowledgements
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (no. 51402253) and the Independent Research Program for the Young Teachers of Yanshan University (no. 14LGA007).Notes and references
- C. H. An, S. Peng and Y. G. Sun, Adv. Mater., 2010, 22, 2570 CrossRef CAS PubMed.
- F. Z. Liu, Y. H. Leung, A. B. Djurišić, A. M. C. Ng and W. K. Chan, J. Phys. Chem. C, 2013, 117, 12218 CAS.
- R. Gong, J. J. Ye, W. Dai, X. Y. Yan, J. Hu, X. Hu, S. Li and H. Huang, Ind. Eng. Chem. Res., 2013, 52, 14297 CrossRef CAS.
- N. Bao, Y. Li, Z. T. Wei, G. B. Yin and J. J. Niu, J. Phys. Chem. C, 2011, 115, 5708 CAS.
- X. W. Zhang, D. K. Wang, D. R. S. Lopez and J. C. D. da Costa, Chem. Eng. J., 2014, 236, 314 CrossRef CAS.
- L. Tan, H. Li, S. X. Ning and B. W. Xu, Bioresour. Technol., 2014, 158, 321 CrossRef CAS PubMed.
- K. Yu, S. G. Yang, C. Liu, H. Z. Chen, H. Li, C. Sun and S. A. Boyd, Environ. Sci. Technol., 2012, 46, 7318 CrossRef CAS PubMed.
- Z. S. Liu, H. S. Ran, J. N. Niu, P. Z. Feng and Y. B. Zhu, J. Colloid Interface Sci., 2014, 431, 187 CrossRef CAS PubMed.
- T. M. Breault and B. M. Bartlett, J. Phys. Chem. C, 2013, 117, 8611 CAS.
- Y. J. Zhu, Y. Q. Sun, X. Y. Niu, F. L. Yuan and H. G. Fu, Catal. Lett., 2010, 135, 152 CrossRef CAS.
- H. B. Sales, V. Bouquet, S. Députier, S. Ollivier, F. Gouttefangeas, G. V. Maryline, V. Dorcet, I. T. Weber, A. G. de Souza and I. M. G. dos Santos, Solid State Sci., 2014, 28, 67 CrossRef CAS.
- J. F. Cao, Y. X. Ji, C. B. Tian and Z. G. Yi, J. Alloys Compd., 2014, 615, 243 CrossRef CAS.
- L. Q. Fan, Y. L. Wei, Y. H. Cheng, Y. F. Huang, S. C. Hao and J. H. Wu, Int. J. Hydrogen Energy, 2014, 39, 7747 CrossRef CAS.
- D. Y. Yoon, E. Lim, Y. J. Kim, J. H. Kim, T. Ryu, S. Lee, B. K. Cho, I. S. Nam, J. W. Choung and S. Yoo, J. Catal., 2014, 319, 182 CrossRef CAS.
- K. B. Li, C. X. Dong, Y. H. Zhang, H. Wei, F. Zhao and Q. Q. Wang, J. Mol. Catal. A: Chem., 2014, 394, 105 CrossRef CAS.
- J. M. Du, J. L. Zhang, Z. M. Liu, B. X. Han, T. Jiang and Y. Huang, Langmuir, 2006, 22, 1307 CrossRef CAS PubMed.
- X. Liu, Z. Q. Liu, J. L. Lu, X. L. Wu, B. Xu and W. Chu, Appl. Surf. Sci., 2014, 288, 513 CrossRef CAS.
- J. Lu, H. H. Wang, S. J. Dong, F. Q. Wang and Y. F. Dong, J. Alloys Compd., 2014, 617, 869 CrossRef CAS.
- S. W. Lee, L. M. L. Sánchez and V. R. González, J. Hazard. Mater., 2013, 263, 20 CrossRef CAS PubMed.
- X. B. Li, S. W. Yang, J. Sun, P. He, X. G. Xu and G. Q. Ding, Carbon, 2014, 78, 38 CrossRef CAS.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132 CrossRef CAS PubMed.
- Q. J. Xiang, J. G. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782 RSC.
- Z. Y. Ji, X. P. Shen, J. L. Yang, Y. L. Xu, G. X. Zhu and K. M. Chen, Eur. J. Inorg. Chem., 2013, 6119 CrossRef CAS.
- S. F. Wang, G. Xue, J. S. Liang, Y. K. Yuan and X. L. Zhang, Catal. Commun., 2014, 45, 39 CrossRef CAS.
- S. Y. Dong, Y. R. Cui, Y. F. Wang, Y. K. Li, L. M. Hu, J. Y. Sun and J. H. Sun, Chem. Eng. J., 2014, 249, 102 CrossRef CAS.
- X. Y. Zhang, H. P. Li, X. L. Cui and Y. H. Lin, J. Mater. Chem., 2010, 20, 2801 RSC.
- Q. J. Xiang, J. G. Yu and M. Jaroniec, J. Am. Chem. Soc., 2012, 134, 6575 CrossRef CAS PubMed.
- L. X. Jiang, K. X. Li, L. S. Yan, Y. H. Dai and Z. M. Huang, Chin. J. Catal., 2012, 33, 1974 CAS.
- K. X. Li, Y. Huang, L. S. Yan, Y. H. Dai, K. P. Xue, H. Q. Guo, Z. M. Huang and J. J. Xiong, Catal. Commun., 2012, 18, 16 CrossRef CAS.
- K. Nawaz, U. Khan, N. U. Haq, P. May, A. O’Neill and J. N. Coleman, Carbon, 2012, 50, 4489 CrossRef CAS.
- Q. J. Xiang, J. G. Yu and P. K. Wong, J. Colloid Interface Sci., 2011, 357, 163 CrossRef CAS PubMed.
- B. Thongrom, P. Amornpitoksuk, S. Suwanboon and J. Baltrusaitis, Korean J. Chem. Eng., 2014, 31, 587 CrossRef CAS.
- Q. J. Xiang, K. L. Lv and J. G. Yu, Appl. Catal., B, 2010, 96, 557 CrossRef CAS.
- T. Tachikawa and T. Majima, J. Am. Chem. Soc., 2009, 131, 8485 CrossRef CAS PubMed.
- L. H. Ai, C. H. Zhang and J. Jiang, Appl. Catal., B, 2013, 142–143, 744 CrossRef CAS.
- L. Xu, H. M. Zang, Q. Zhang, Y. Chen, Y. G. Wei, J. H. Yan and Y. H. Zhao, Chem. Eng. J., 2013, 232, 174 CrossRef CAS.
- X. D. Zhu, Y. J. Wang, R. J. Sun and D. M. Zhou, Chemosphere, 2013, 92, 925 CrossRef CAS PubMed.
- S. N. Zhang, S. J. Zhang, L. M. Song, X. Q. Wu and S. Fang, Chem. Eng. J., 2014, 243, 24 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |



