Molecularly imprinted polymer-based sensors for atrazine detection by electropolymerization of o-phenylenediamine†
Xiao Li,
Yanfen He,
Fan Zhao,
Weiying Zhang* and
Zhuoliang Ye
School of Chemical Engineering, Fuzhou University, Fuzhou 350108, China. E-mail: wyzhang@fzu.edu.cn
First published on 19th June 2015
Abstract
A sensitive and selective atrazine (ATZ) electrochemical sensor was developed based on a molecularly imprinted polymer (MIP). Density functional theory at the B3LYP/6-31G (d,p) level and the Gaussian 2009 package was used to calculate the interaction energy of template-monomers. The MIP sensing film was prepared by electropolymerization of o-phenylenediamine (o-PD) using ATZ as the template. Some factors affecting the activity of the sensor have been discussed. The performance of the sensor was characterized by cyclic voltammetry and differential pulse voltammetry. Under the optimal experimental conditions, the relative current change was linear to the concentration of ATZ in the range of 5.0 × 10−9 to 1.4 × 10−7 M with a detection limit of 1.0 × 10−9 M. The result of the selectivity experiment showed that the imprinted electrode has a good response and selectivity towards ATZ. The proposed MIP-based sensor also showed good stability and repeatability.
1. Introduction
Atrazine (ATZ) is a conventional triazine herbicide which has been widely used in the annual control of some grasses and broadleaf weeds in corn, sugar cane, sorghum, and other cultures.1,2 ATZ has strong lethality for weeds and its price is cheap. But after a long period of study, it has been found that ATZ is harmful to people and animals. Therefore, ATZ is classified as a priority pollutant which is currently prohibited according to the EC legislation (Commission Decision 2004/248/EC). ATZ has good water solubility, a long residual period, and can exist in the environment for a long time.3–5 Thus, the detection of ATZ has become more and more important.Techniques to determine trace amount of ATZ in the environment include spectrophotometric,6,7 high performance liquid chromatography (HPLC)8–10 and gas chromatography (GC).11,12 However, these techniques require complicated sample preparation usually involving pre-concentration, which is expensive and time-consuming. Thus, a simple, highly sensitive and selective method for trace ATZ detection is highly desirable in routine analytical practice.
Electrochemical sensors have the advantages of high sensitivity, simple operation and low cost. It has been used in detection of environmental pollutants.13 The core of electrochemical sensors is recognition elements which can identify and combine with the specific target analytes. MIP is highly selective functional materials. The structure of MIP has many imprinted cavities that match with template molecule in shape and size.14 MIP has been widely used in chromatographic separation, solid-phase extraction, enzyme-mimic catalysis and molecular recognition sensors.15–19 Recently, Molecularly imprinted electrochemical sensors (MIECS) which use MIP as the recognition element has attracted extensive attention.20,21 MIP has several advantages over biologically recognition element, including high affinity, high selectivity, and inexpensive.
There have been many reports about ATZ detection by MIPs based electrochemical sensors.22–24 Pardieu et al.25 have developed an electrochemical sensor based on molecularly imprinted conducting polymer for detection of ATZ. The MICP, poly(3,4-ethylenedioxythiophene-co-thiophene-acetic acid), has been electrochemically synthesized onto a platinum electrode. The developed sensor showed a good selectivity for ATZ and low detection limit (10−7 M). Liu et al.26 have developed a core–shell nanostructure molecular imprinting fluorescent chemosensor for selective detection of ATZ. The sensor showed a detection limit (LOD) of about 1.8 μM and high selectivity for ATZ. o-Phenylenediamine (o-PD) is easy to be electropolymerized on various substrates to form films with good chemical and mechanical stability.27–29 There were many preparations of MIECS with o-PD as functional monomer was reported.30,31 This method has the advantage of easy and fast, which don't need a crosslinking agent and solvent.
In this work, the interaction between o-PD and ATZ was calculated by using the density functional theory (DFT) method in order to optimize the ratio of ATZ to o-PD. MIP film was then prepared by electropolymerizing o-PD in the presence of ATZ, and an ATZ electrochemical sensor based on MIP sensing film was developed. The developed sensor showed specific recognition toward ATZ with a rather low detection limit of 1.0 × 10−9 M.
2. Experimental
2.1. Materials
Atrazine, simazine and alachlor were bioreagent purchased from Shandong Qiaochang Chemical Co., Ltd. (China). o-Phenylenediamine, potassium ferricyanide and potassium ferrocyanide were analytical grade obtained from Sinopharm Chemical Reagent Company (China). All other reagents were of analytical grade and were used without further purification. Phosphate buffer solution (PBS, pH 7.4) was prepared by mixing 0.1 M Na2HPO4 and NaH2PO4. All solutions were prepared with D.I. water.2.2. Apparatus
Electrochemical measurements were performed on a CHI660 electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd., China) coupled with a conventional three-electrode cell. The three-electrode system consists of a saturated calomel electrode as the reference electrode, a platinum wire electrode as the auxiliary electrode and a bare or polymer-modified gold electrode (2 mm in diameter) as the working electrode.2.3. Computational calculation
All calculations were carried out using Gaussian 09 software package. Conformation optimization of ATZ, o-PD and their complexes was performed by DFT at B3LYP/6-31G (d,p) level. The electronic stabilization energy, ΔE, was calculated through eqn (1).| ΔE = E(ATZ-o-PD complex) − E(ATZ) − ∑E(o-PD) | (1) |
2.4. Preparation of MIP-based sensor
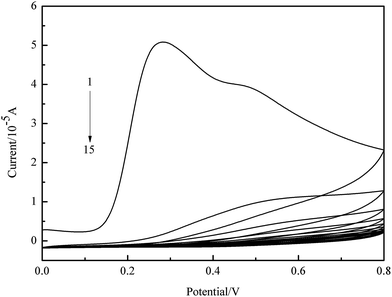
Molecular self-assembly was achieved by mixing 1 mM ATZ and 5 mM o-PD in PBS (pH = 7.4) for 5 h. Gold electrode surface was polished to a mirror finish using aqueous slurry of 0.05 μm alumina particles before washed with water and ethanol in an ultrasonic cleaner. Then the electrode was subjected to cyclic potential sweeps between −0.1 to 1.4 V in 0.5 M H2SO4 until stable cyclic voltammograms was obtained. Next, MIP was prepared on the gold electrode by electropolymerization in the self-assembled solution. Fifteen cycles of cyclic voltammograms were performed in the potential range of 0–0.8 V (scan rate 50 mV s−1). The gold electrode with MIP film was then washed in the mixed solvent of methanol/acetic acid (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) to remove the templates. A MIP-based sensor with stereo cavities in imprinted membranes was obtained. The scheme of preparation was showed in Fig. 1.
1 v/v) to remove the templates. A MIP-based sensor with stereo cavities in imprinted membranes was obtained. The scheme of preparation was showed in Fig. 1.
A gold electrode with non-imprinted film was prepared following the same procedure as above but in the absence of ATZ.
2.5. Electrochemical measurement
All the electrochemical measurements were carried out in 5 mM K3[Fe(CN)6]/K4[Fe(CN)6] solution containing 0.1 M KCl at room temperature. Cyclic voltammetry (CV) measurements were performed over a potential range from −0.1 V to 0.5 V with a scan rate of 50 mV s−1. Differential pulse voltammetry (DPV) measurements were conducted from −0.1 V to 0.5 V. The amplitude, width and period of pulse were 50 mV, 50 ms and 200 ms, respectively. Before electrochemical measurements, the solution was purged with N2 for 10 min.2.6. Determination of ATZ
DPV measurements were performed in 5 mM K3[Fe(CN)6]/K4[Fe(CN)6] solution containing 0.1 M KCl after the imprinted electrode was incubated in aqueous solutions containing different concentrations of ATZ for 8 min. Relative current change (ΔI/I0) was employed to characterize the adsorption of modified electrode for ATZ. Herein, I0 is the peak current in background solution and ΔI is the difference between I0 and the peak current in the presence of ATZ.2.7. Selectivity toward ATZ
The selectivity of the MIP-based sensor for ATZ was tested by DPV. The selectivity factor, α, was used to represent the selectivity and was calculated according to eqn (2).| α = (ΔI/I0)ATZ/(ΔI/I0)interferent | (2) |
Simazine and alachlor were chosen as the interferents. The structures of ATZ, simazine and alachlor were showed in Fig. 2.
3. Results and discussion
3.1. Computational simulation of ATZ and o-PD complex
The configurations of ATZ, o-PD and ATZ-o-PD complexes were constructed and optimized by DFT at B3LYP level with 6-31G (d,p). The results were shown in Fig. 3. The binding energy between ATZ and o-PD with different ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was calculated and the results were summarized in Table 1. A complex with higher absolute value of ΔE predicts a more stable configuration. As it was observed, the absolute value of ΔE increased with increasing proportion of o-PD. When the ratio of ATZ to o-PD was 1
4) was calculated and the results were summarized in Table 1. A complex with higher absolute value of ΔE predicts a more stable configuration. As it was observed, the absolute value of ΔE increased with increasing proportion of o-PD. When the ratio of ATZ to o-PD was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the binding energy had the minimum value of −92.682 kJ mol−1, and the complex was the most stable. When the ratio was 1
4, the binding energy had the minimum value of −92.682 kJ mol−1, and the complex was the most stable. When the ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, stable complex configuration wasn't obtained by calculation. This might be attributed to the steric hindrance, resulting in the increasing repulsive force between ATZ and o-PD. The distance between molecules increased and the active sites of hydrogen bond reduced. The stability of complex became weak.
5, stable complex configuration wasn't obtained by calculation. This might be attributed to the steric hindrance, resulting in the increasing repulsive force between ATZ and o-PD. The distance between molecules increased and the active sites of hydrogen bond reduced. The stability of complex became weak.
 | ||
| Fig. 3 Optimized conformations of ATZ, o-PD and their complexes by DFT. (a) ATZ; (b) o-PD; (c) ATZ + o-PD; (d) ATZ + 2o-PD; (e) ATZ + 3o-PD; (f) ATZ + 4o-PD. | ||
| Mole ratio of ATZ to o-PD | E (Hartree) | ΔE (Hartree) | ΔE (kJ mol−1) |
|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
−1047.310225 | — | — |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−342.975005 | — | — |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−1390.299518 | −0.01428845 | −37.51432548 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
−1733.283434 | −0.02319904 | −60.90907952 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
−2076.265512 | −0.03027203 | −79.47921477 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
−2419.245546 | −0.03530073 | −92.68206662 |
3.2. Electropolymerization for MIP-based sensors
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 1
4 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 while the concentration of o-PD kept 5 mM. Fig. 5(B) showed the relative current change of imprinted electrodes with different ratios of ATZ to o-PD. The great relative current change appeared at the ratio of 1
8 while the concentration of o-PD kept 5 mM. Fig. 5(B) showed the relative current change of imprinted electrodes with different ratios of ATZ to o-PD. The great relative current change appeared at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. When the ratio was less than 1
5. When the ratio was less than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the quantity of available binding sites reduced due to the lower concentration of ATZ. Moreover, the excessive o-PD might lead to increase of non-selective binding sites generated by surplus o-PD, and decrease of the specific adsorption to ATZ. When the ratio was more than 1
5, the quantity of available binding sites reduced due to the lower concentration of ATZ. Moreover, the excessive o-PD might lead to increase of non-selective binding sites generated by surplus o-PD, and decrease of the specific adsorption to ATZ. When the ratio was more than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the amount of functional monomer was not enough to combine all the template molecules. The molar ratio of 1
5, the amount of functional monomer was not enough to combine all the template molecules. The molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 was considered suitable for production of MIP film. It is somewhat inconsistent with the result of computational modeling. The reason was that there was electrostatic attraction between ATZ and o-PD besides hydrogen bond.
5 was considered suitable for production of MIP film. It is somewhat inconsistent with the result of computational modeling. The reason was that there was electrostatic attraction between ATZ and o-PD besides hydrogen bond.3.3. Electrochemical characterization of MIP and NMIP electrodes
Cyclic voltammograms of different electrodes in 5 mM K3[Fe(CN)6]/K4[Fe(CN)6] solution containing 0.1 M KCl were shown in Fig. 6(A). Curve a showed a pair of typical redox peaks of ferricyanide probe occurring on the surface of the bare gold electrode. From curve a to b, the peak current decreased to near zero, which indicated that a nonconductive polymer film was formed on the electrode surface and hindered the redox probe from getting access to the surface of the electrode. After the removal of ATZ, the peak current increased (curve c). It showed that ATZ molecules were eluted from the polymer film, leaving cavities and the probe obtained the access to the electrode surface. When the imprinted film rebound ATZ, the cavities were again blocked and the peak current decreased (curve d). But it was higher than that of curve b, because some cavities might be deformed to hinder the entrance of ATZ molecules.As for the NMIP electrode (Fig. 6(B)), the peak current decreased greatly from curve a to e, because the poly (o-PD) film in the absence of ATZ covered the surface of the gold electrode. The change of current was negligible from curve e to f, because no imprinted cavities were obtained after the NMIP electrode being washed with the mixed solvent of methanol/acetic acid.
3.4. Detection range of ATZ
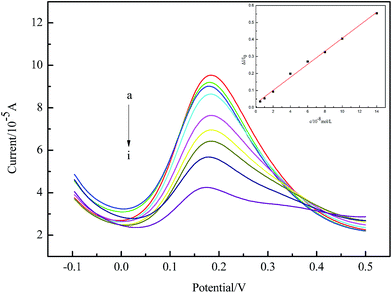
The DPV current responses of the imprinted electrode to the concentrations of ATZ were displayed in Fig. 7. The peak current decreased with increasing ATZ concentration. The inset in Fig. 7 showed the relationship between the relative change of peak current and the concentration of ATZ. The relative current change increased linearly with the concentration of ATZ between 5.0 × 10−9 and 1.4 × 10−7 M. The linear regression curve was shown as eqn (3) with a coefficient of correlation of 0.998. And the detection limit was 1.0 × 10−9 M.| ΔI/I0 = 0.03831c (10−8 M) + 0.02422 | (3) |
3.5. Selectivity of imprinted electrode
In order to check the specificity of recognition of the MIP-based sensor towards ATZ, simazine and alachlor were chosen as the interferents. The responses to simazine and alachlor of the imprinted electrode were measured by DPV. The relative change of peak current to simazine and alachlor were showed in Fig. 8 (the data was shown in Table S1, ESI†). The results showed that ATZ produced higher current changes than simazine, which meant that there was a weak recognition of simazine in comparison with ATZ. The reason was that both simazine and ATZ belong to triazine family and present two side-chain amino groups. Therefore, simazine may establish hydrogen-bonds with the nitrogen and hydrogen atoms of poly(o-PD) at the surface of MIP cavities. Alachlor, however, produced almost negligible current changes due to its fundamentally different structure compared to ATZ. This pesticide did not match the functionalized cavities of MIP film even if it could establish hydrogen-bonds. The recognition of the NIP-based sensor towards ATZ and its analogue were explored as MIP-based sensor, but the result showed that the NIP-based sensor have no response to ATZ. | ||
| Fig. 8 Relative current change of imprinted electrode to ATZ and analogues at different concentrations. | ||
Table 2 showed that selectivity factors of MIP-sensors toward different concentrations of interferents were more than 1, so the MIP-sensors have selectivity for ATZ.
| Concentration/10−8 M | α | |
|---|---|---|
| Simazine | Alachlor | |
| 2 | 1.66 | 1.19 |
| 4 | 1.60 | ∞ |
| 6 | 2.07 | ∞ |
| 8 | 2.49 | ∞ |
| 10 | 3.00 | ∞ |
As a conclusion, the developed MIP sensor displays a selective recognition towards ATZ.
3.6. Stability and repeatability
To ensure the stability, the imprinted electrode was stored at room temperature for a period of time before being used. The current response decreased about 3.6% after a week, and decreased about 13.3% after two weeks. It was demonstrated that the imprinted electrode had fairly good stability. The repeatability of the imprinted electrodes was investigated by detecting 1 × 10−8 M of ATZ. The calculated RSD in six parallel tests was about 5.43%. This result confirmed that the sensor had good test repeatability.The recovery of imprinted electrode also was studied and the data was shown in Table 3. The results showed that the recovery of imprinted electrode ranged from 95.5% to 103.5%.
| Added (10−8 mol L−1) | Found (10−8 mol L−1) | Recovery (%) |
|---|---|---|
| 2.00 | 2.07 | 103.5 |
| 2.00 | 1.91 | 95.5 |
| 2.00 | 1.94 | 97.0 |
| 4.00 | 3.86 | 96.5 |
| 4.00 | 3.89 | 97.3 |
| 4.00 | 3.93 | 98.3 |
| 8.00 | 7.92 | 99.0 |
| 8.00 | 7.88 | 98.5 |
| 8.00 | 8.12 | 101.5 |
4. Conclusions
A MIP-based electrochemical sensor for ATZ was successfully constructed by the electropolymerization of o-PD on the surface of a gold electrode in the presence of ATZ. The specific imprinted electrode showed sensitivity and selectivity toward ATZ. The MIP-based sensor exhibited a rather low detection limit down to 1 × 10−9 M in a linear detection range from 5.0 × 10−9 to 1.4 × 10−7 M. The sensor showed good stability and repeatability.Acknowledgements
This work was financially supported by the Natural Science Foundation of Fujian Province of China (no. 2010J01037) and the Science and Technology Guiding Projects of Fujian Province (no. 2015H0019).References
- L. Svorc, M. Rievaj and D. Bustin, Sens. Actuators, B, 2013, 181, 294–300 CrossRef CAS PubMed.
- K. Prasad, K. P. Prathish, J. Mary Gladisa, G. R. K. Naidu and T. Prasada Rao, Sens. Actuators, B, 2007, 123, 65–70 CrossRef CAS PubMed.
- H. Xing, X. Wang, G. Sun, X. Gao, S. Xu and X. Wang, Environ. Toxicol. Pharmacol., 2012, 33, 233–244 CrossRef CAS PubMed.
- M. Kucka, K. Pogrmic-Majkic, S. Fa, S. S. Stojilkovic and R. Kovacevic, Toxicol. Appl. Pharmacol., 2012, 265, 19–26 CrossRef CAS PubMed.
- J. G. Gu, Y. Z. Fan and J. D. Gu, Chemosphere, 2003, 52, 1515–1521 CrossRef CAS.
- G. Zhang and J. Pan, Spectrochim. Acta, Part A, 2011, 78, 238–242 CrossRef PubMed.
- E. Ayranci and N. Hoda, J. Hazard. Mater., 2004, 112, 163–168 CrossRef CAS PubMed.
- Z. Kuklenyik, P. Panuwet, N. K. Jayatilaka, J. L. Pirkle and A. M. Calafat, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2012, 901, 1–8 CrossRef CAS PubMed.
- R. X. Mou, M. X. Chen, Z. Y. Cao and Z. W. Zhu, Anal. Chim. Acta, 2011, 706, 149–156 CrossRef CAS PubMed.
- M. T. Muldoon and L. H. Stanker, Anal. Chem., 1997, 69, 803–808 CrossRef CAS.
- B. D. Real, M. C. Ortiz and L. A. Sarabia, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2012, 910, 122–137 CrossRef CAS PubMed.
- I. K. Konstantinou, T. M. Sakellarides, V. A. Sakkas and T. A. Albanis, Environ. Sci. Technol., 2001, 35, 398–405 CrossRef CAS.
- M. J. Schöning, R. Krause and K. Block, Sens. Actuators, B, 2003, 95, 291–296 CrossRef.
- P. A. Cormack and A. Z. Elorza, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, 804, 173–182 CrossRef CAS PubMed.
- F. Lanza and B. Sellergren, Chromatographia, 2001, 53, 599–611 CAS.
- X. F. Zheng, Q. Lian and H. Yang, RSC Adv., 2014, 4, 42478–42485 RSC.
- Q. Fu, H. Sanbe, C. Kagawa, K. K. Kunimoto and J. Hagina, Anal. Chem., 2003, 75, 191–198 CrossRef CAS.
- G. Wulff, Chem Rev., 2002, 102, 1–27 CrossRef CAS PubMed.
- C. M. Dai, X. F. Zhou and Y. L. Zhang, J. Hazard. Mater., 2011, 198, 175–181 CrossRef CAS PubMed.
- T. Alizadeh and S. Amjadi, J. Hazard. Mater., 2011, 190, 451–459 CrossRef CAS PubMed.
- W. R. Huang, Y. L. Chen, C. Y. Lee and H. T. Chiu, RSC Adv., 2014, 4, 62393–62398 CAS.
- R. Shoji, T. Takeuchi and I. Kubo, Anal. Chem., 2003, 75, 4882–4886 CrossRef CAS.
- T. A. Sergeyeva, O. V. Piletska, S. A. Piletsky, L. M. Sergeeva, O. O. Brovko and G. V. El'ska, Mater. Sci. Eng., C, 2008, 28, 1472–1479 CrossRef CAS PubMed.
- D. Lakshmi, M. Akbulut, P. K. Ivanova-Mitseva, M. J. Whitcombe, E. V. Piletska, K. Karim, O. Guven and S. A. Piletsky, Ind. Eng. Chem. Res., 2013, 52, 13910–13916 CrossRef CAS.
- E. Pardieu, H. Cheap, C. Vedrine, M. Lazerges, Y. Lattach, F. Garnier, S. Remita and C. Pernelle, Anal. Chim. Acta, 2009, 649, 236–245 CrossRef CAS PubMed.
- R. Liu, G. Guan, S. Wang and Z. Zhang, Analyst, 2011, 136, 184 RSC.
- Z. L. Cheng, E. K. Wang and X. R. Yang, Biosens. Bioelectron., 2011, 6, 179–185 Search PubMed.
- J. P. Li, S. H. Li, X. P. Wei, H. L. Tao and H. C. Pan, Anal. Chem., 2012, 84, 9951–9955 CrossRef CAS PubMed.
- Y. Liu, Q. J. Song and L. Wang, Microchem. J., 2009, 91, 222–226 CrossRef CAS PubMed.
- X. Wen, X. Li, W. Y. Zhang and X. G. Ying, Chem. Res. Chin. Univ., 2012, 33, 2199–2204 Search PubMed.
- Z. Cheng, E. Wang and X. Yang, Biosens. Bioelectron., 2001, 16, 179–185 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09556e |
| This journal is © The Royal Society of Chemistry 2015 |