Applications of supramolecular capsules derived from resorcin[4]arenes, calix[n]arenes and metallo-ligands: from biology to catalysis
Chiara M. A. Gangemi
a,
Andrea Pappalardo
*ab and
Giuseppe Trusso Sfrazzetto
*a
aDepartment of Chemical Sciences, University of Catania, Viale A. Doria 6, 95125 Catania, Italy. E-mail: andrea.pappalardo@unict.it; giuseppe.trusso@unict.it
bI.N.S.T.M. UdR of Catania, Viale A. Doria 6, 95125 Catania, Italy
First published on 5th June 2015
Abstract
Supramolecular architectures developed after the initial studies of Cram, Lehn and Pedersen have become structurally complex but fascinating. In this context, supramolecular capsules based on resorcin[4]arenes, calix[n]arenes or metal–ligand structures are dynamic assemblies inspired by biological systems. The reversible formation of these assemblies, combined with the possibility to modify their dimensions and shapes in the presence of a guest (concepts of reversibility and adaptivity) make them similar to biological macromolecules, such as proteins and enzymes. The small space inside a supramolecular capsule is characterized by different properties compared to the bulk solution. This review describes concrete applications of capsular supramolecular self-assemblies in the biomedical field, in catalysis and in material science.
Introduction
The enhancements in the noncovalent synthesis of macrocyclic molecules have led to the development of supramolecular chemistry, a field that has attracted increasing attention in the last few decades.1–7 Many important aspects have been investigated by supramolecular chemistry including molecular self-assembly,8 folding,9 molecular recognition, host–guest chemistry,10 mechanically-interlocked molecular architectures,11 and dynamic covalent chemistry.2 Researchers have explored the host–guest interactions by employing several classical macrocyclic hosts, comprising crown ethers, cyclodextrins, calixarenes, cavitands and cucurbiturils. Since the pioneering studies of Donald J. Cram, Jean-Marie Lehn and Charles J. Pedersen (Nobel Prizes for Chemistry in 1987), researchers started to survey exciting areas of material chemistry and nanoscience, with many potential applications in polymer science,12 organic photovoltaics,13 as well as in vitro and in vivo biological imaging.14 In particular, supramolecular chemistry built on weak and reversible non-covalent interactions has emerged as a powerful and versatile strategy for the fabrication of new materials, due to its facile accessibility, extraordinary reversibility and adaptivity.Supramolecular capsules represent a branch of these new materials: the inner space inside them shows unique properties, different from the bulk solution, making them new attractive and useful “supramolecular tools” in different fields. Cram's work was concerned with the formation of molecular capsules (carcerands or hemicarcerands), by covalently linking two resorcin[4]arene units.15 The high stability of these capsules associated with the covalent nature of their formation, represent a main advantage. However, the low yielding syntheses and the difficulty to encapsulate/release a specific guest have compelled the researchers to develop a different strategy to obtain containers, able to self-assembly in solution, thus leading to the “supramolecular capsules”. Hydrogen bonding is one of the strongest driving forces for a well-defined self-aggregation. Rebek et al. firstly reported on the formation of a supramolecular capsule, exploiting the formation of several hydrogen bonds between two bis-glycoluril derivatives.16 A remarkable key aspect of the supramolecular strategy to generate a capsules is the dynamic nature of the self-assembly, which leads to the mutual modification of the molecular structures of host and guest.17–21 As a consequence, physical–chemical properties of guests included inside a container are altered with respect to the free molecules in solution. For these reasons, supramolecular cages have found applications in different fields, as alternative route to the classic chemistry in solution.
A fundamental contribute to the study and characterization of supramolecular capsules was provided by the development of sophisticated NMR techniques, particularly diffusion NMR.22–27 Diffusion ordered spectroscopy (DOSY)28 was used to characterize and determine dimensions and structure of several host–guest systems,29–39 organometallic complexes,40–44 supramolecular systems,45–49 and supramolecular polymers.50–53 Recently, Cohen and Avram reported on the applications of the diffusion NMR to study cages and container molecules, describing some methodological and practical aspects.54 In general, three strategies for the self-assembly of supramolecular capsules are employed: hydrogen bonding, metal coordination and hydrophobic effect. The success of these approaches is warranted by the strong and highly directional non-covalent interactions that lead to the product formation. However, the inclusion of a guest inside the capsule facilitates the self-assembly process. As a result of the formation of supramolecular complex, encapsulated guests should diffuse as a single entity with the capsule, causing a clear change of the diffusion coefficient of the included guest. An additional significant contribute for the understanding of supramolecular systems was also given by Dynamic Light Scattering techniques (DLS).55
This review collects the applications of supramolecular capsules assembled from calixarenes, resorcinarenes and metallo-cage building blocks. In particular, the applications of these capsular supramolecular assemblies as nanoreactors for specific organic reactions are analyzed and discussed, without neglecting the applications of some hosts as new materials in AFM analysis and in the biomedical field as drug delivery systems.
Biomedical applications
Calix[n]arenes and cavitands have been used for biomedical applications,56 albeit they are macrocyclic hosts that show hydrophobic properties and poor water solubility. To overcome this drawback, researchers have spent considerable effort to prepare hydrophilic and water-soluble hosts. For example in calix[n]arene derivatives, sulfonation,57 the introduction of carboxylic acid groups at the lower rim,58 and the functionalization with polar groups at the upper rim59 are the most common strategies to prepare water-soluble hosts. Furthermore, by using click chemistry, cationic, anionic, and non-ionic hydrophilic calixarene macrocycles have been efficiently synthesized.60 In terms of biocompatibility, water-soluble calixarene derivatives exhibit low toxicity, and the in vivo dosage can reach up to 100 mg kg−1 without any toxic effect in mice.61 On the basis of these considerations, the formation of supramolecular structures capable of encapsulating guest molecules into the host cavities has become an attractive topic. Although biomedical applications of cyclodextrins are widely diffused,62 no reports have so far appeared in the literature concerning concrete applications of cyclodextrin capsules. Furthermore, the binding constants of calix[n]arenes and resorcin[4]arene cavitands toward guest molecules are usually higher than those with cyclodextrins. In this context, Nau and co-workers reported on the possibility to encapsulate guest drugs into calixarene derivatives.63Supramolecular approach has been used in drug delivery, mainly to solve the problems associated with the low solubility of hydrophobic drugs in aqueous solution, and the difficulty of anticancer drugs to penetrate the cancer cell membranes.64–66 Chemical activity of drugs might be improved by their inclusion into the inner space of a capsule and, upon external stimuli, (i.e. thermal change, pH variation, or competitive binding) the encapsulated drugs can be released from the cavity, leading to prolonged therapeutic effects.67
Drug delivery
Menon and co-workers prepared a para-sulfonatocalix[4]resorcinarene 1 able to encapsulate several drugs including the poorly soluble mycophenolate mofetil – an immunosuppressant drug used to prevent rejection in organ transplantation – by forming the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes (Fig. 1).68–70
1 host–guest complexes (Fig. 1).68–70
 | ||
| Fig. 1 Drugs used by Menon and co-workers to form the host–guest complexes with para-sulfonatocalix[4]resorcinarene 1. | ||
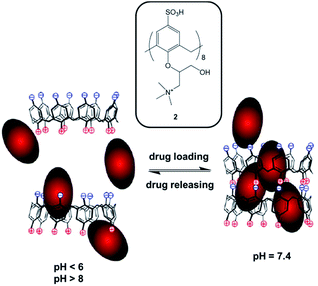
Xiao reported on a new amphoteric calix[8]arene 2 that displays a pH-triggered drug releasing behavior (Scheme 1).71 The upper rim of the calix[8]arene is functionalized with sulfonate groups, while the lower rim presents positive charges due to the presence of quaternary ammonium groups.
Hydrophobic model drug, ciprofloxacin, was included into the calixarene cavity at neutral pH, self-assembling into superstructures through electrostatic interactions between the upper and lower rims (Scheme 1). At higher or lower pH values, the capsule disassembles releasing the ciprofloxacin. Such pH-responsive drug delivery system shows great potential for future theranostic applications.
Very recently, Lippard et al. reported on an innovative application of the tetrahedral metal-cage 3 (M = Pt) as nanocontainer of a cis-platinum prodrug able to exhibit high cellular uptake (Fig. 2 and Scheme 2).72
The metallo-cage 3, reported by Fujita in 1995, is one of the first capsular systems based on the metal coordination, assembled from four tridentate nitrogen-based ligands, six metal (palladium or platinum) ions, and six ethylendiamine ligands.73 This cage can accommodate several guests, including tripeptides via a series of π–π and CH–π interactions and intramolecular guest–guest interactions.74–77
In particular, the delivery systems constituted by cytotoxic Pt(IV)-prodrugs and the hexanuclear Pt(II) cage 3 possess low toxicity, and an excellent cellular uptake due to the presence of twelve positive charges (Scheme 2).
To explicate anticancer activity, cisplatin must be released from this host–guest system upon reduction with ascorbic acid. The supramolecular cage 3 displays micromolar potency, comparable to cisplatin, against several cancer cell lines (lung carcinoma, ovarian carcinoma, and ovarian carcinoma).
Applications in catalysis
Metallo-cage capsules
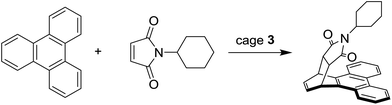
The inclusion of two molecules within such a small volume leads to a high concentration of the guests and, consequently, to an increased likelihood of reaction between them. Fujita and co-workers used metallo-cage 3 to catalyze reactions of normally unreactive species, or to induce asymmetric reactions into the cavity of the cage, which possesses a chiral element attached to the metal center.78 The enhancement of “activity” of the guests inside a similar supramolecular cage is demonstrated by the Diels–Alder reaction between the triphenylene and maleimides shown in Scheme 3,79 and by the photocatalytic reaction between o-quinone and 4-(1-adamantyl)-toluene reported in Scheme 4.80Ramamurthy et al. reported also on the reaction of photodimerization of trans-cinnamic acid esters inside the capsular system 3, leading to both the monomeric cis-isomer, in considerably reduced yield with respect to the reaction in absence of the cage 3, and dimeric products in a range of 21–63% yield.81 The same research group has also examined how host 3 can afford the exclusive formation of syn head–head dimers of several coumarin derivatives, while in free aqueous solution syn head–head, syn head–tail, and anti head–head dimers are obtained.82 Photodimerization was also investigated using acenaphthylene as guest.83
Raymond's group developed a new tetrahedral metallocage 4 consisting of a series of metal ions (e.g. Ga(III), Al(III), In(III), Ti(IV) and Ge(IV)) and six catecholamide-based ligands (Fig. 3). The metal cation determines the kinetic stability of the capsule, which can include a wide range of guests.84–87
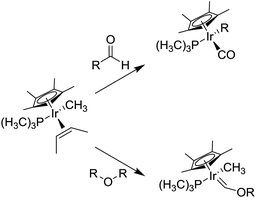
The gallium complex 4 is able to encapsulate in water media the (CpRuCl(cod)) (Cp = cylopentadiene, cod = 1,5-cyclooctadiene), a catalyst used for the formation of C–C bonds.88 Raymond and co-workers studied the applications of tetrahedral metallocage 4 as nanoscale reactor. In particular, the reactivity of encapsulated mono-cationic half-sandwich iridium guests was analyzed (Scheme 5).89 The reactivity of this complex into 4 shows highly specific size and shape selectivities in the C–H bond activation of aldehydes and ethers.
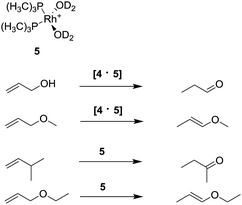
The catalytic properties of other metal transition complexes inside the supramolecular cage 4 were further examined. In particular, a series of bisphosphine rhodium–diene cations were encapsulated, affording the hydrated active catalyst (Rh(PMe3)2(D2O)2) 5 (Scheme 6).90
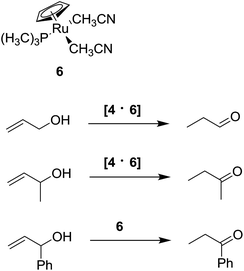
This host–guest complex [4·5] is stable within 12 hours, and catalyzes the isomerization of allylic substrates exhibiting substrate selectivity depending on the steric hindrance (Scheme 6): only prop-2-en-1-ol and its methyl ether were isomerized, while larger substrates are isomerized only by the non-encapsulated catalyst.
Also [RuCp(PMe3)(MeCN)2]+ 6 was encapsulated by 4, leading to the supramolecular catalyst [4·6] capable to isomerize allylic alcohols to the corresponding aldehydes or ketones showing a similar substrates selectivity. In fact, the larger 1-phenylprop-2-en-1-ol does not react with the encapsulated catalyst (Scheme 7).91 Notably, the supramolecular catalyst [4·6] shows a TON higher than those obtained for the non-encapsulated catalyst. The same supramolecular catalyst was used in association with enzymes, like esterases, lipases and alcohol dehydrogenases, to perform cascade reactions. In a one-pot reaction, the aliphatic alcohol was obtained starting from the corresponding allylic alcohol.92 This result demonstrates that metal complex encapsulation can prevent the degradation of the catalyst.
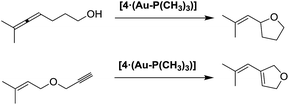 | ||
| Scheme 8 Intramolecular cyclizations of an allen-ol and of an en-yne, catalyzed by supramolecular catalyst [4·(Au–P(CH3)3)]. | ||
Furthermore, supramolecular complex [4·(Au–P(CH3)3)], assembled by the tetrahedral cage 4 and monophosphine gold ion, is able to catalyze the intramolecular cyclization of an allen-ol and of an en-yne (Scheme 8).93,94 The use of the bromide salt of the gold complex in the reaction with allen-ol results in an acceleration rate of eight times, and an increase of TON value of 67. Whereas, in the case of cyclization of en-yne, the formation of the product is selective only in the presence of the supramolecular catalyst, the tetrahedral cage around the gold complex controls the coordination sphere of the gold, acting as a phase transfer reagent, enhancing the reaction rate and tuning the regioselectivity of the reaction.
In addition, Raymond's group reported on the ability of cage 4 to catalyze the 3-aza Cope rearrangements showed in Scheme 9, observing an acceleration of the reaction into the range 5–854 times with respect to the normal conditions.95,96
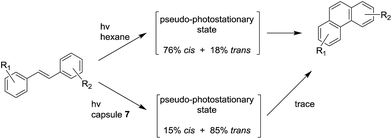 | ||
| Scheme 10 Photochemical products obtained by the photolysis of stilbene derivatives in free solution and inside the OA capsule 7. | ||
Capsule 4 can also accommodate basic amines, phosphines, and (super basic) azaphosphatranes, since these guests are internalized in the protonated state.97 This phenomenon is ascribed to a shift of the pKa values of the guests into the host environment. Searching on a plausible explanation of how host 4 can shift the pKa of an encapsulated guest, Raymond reported on an acid catalysis in basic solution. The formate esters, normally stable in neutral and basic conditions, were rapidly hydrolyzed at pH 11, with a catalytic amount of host 4, showing an acceleration rate of ca. 650–890 times with respect to the non-catalyzed reaction.98 Furthermore, cage 4 has been used as catalyst for the hydrolysis of acetals and ketals at pH 10, with an acceleration rate higher than 1000 times.99,100
Supramolecular capsules assembled via hydrophobic effect
In addition to metal coordination, also hydrophobic effect can induce the formation of supramolecular capsules, exploiting the desolvation of hydrophobic surfaces of the single molecules.101,102 Few examples of self-assembled capsules driven by the hydrophobic effect have been described (compounds 7–10, Fig. 4).103–106 | ||
| Fig. 4 Water soluble cavitands 7–10. Adapted from Z. Laughrey and B. C. Gibb, Chem. Soc. Rev., 2011, 40, 363, with permission of The Royal Society of Chemistry. | ||
The octa acid (OA) derivative 7 is the first example of water soluble deep cavitands based on a resorcin[4]arene scaffold, able to provide supramolecular dimeric capsules in presence of several guests.107 Octa acid cavitands 7–10 differ from similar opened containers (such as micelles, open cavitands, cyclodextrins, cucurbiturils and Pd nanocages) because they are closed containers. Unlike micelles and open cavitands, the entire guest molecule is enveloped within a hydrophobic container. Interestingly, 7 can include propane and butane with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry from the gas phase and the aqueous phase.108 Due to the high difference of binding constant values between propane and butane, capsule 7 can selectively extract butane from a propane/butane mixture. Ramamurthy and co-workers used the OA capsule 7 as chemical reactor in aqueous solution,109 for the generation of singlet oxygen,110,111 the photodimerization of acenaphthylene,112 and the photo-induced rearrangement of dibenzylketone.109,113 Furthermore, this nano-capsule can affect the photochemistry of α-(n-alkyl) dibenzyl ketones depending on the dimension of the guests:114 different kinds of packing motif lead to diverse photochemical behaviors. With the smallest guests a simple decarbonylation is observed, whereas, rearrangement products became prominent with the guests that possess bulkier alkyl groups. To clarify the mechanism of this rearrangement, optically pure α-deoxybenzoins were used as guests contained into OA capsule.115 In addition, analysis with stilbene derivatives as substrate demonstrated that photophysical and photochemical properties of the encapsulated guests are strongly influenced by the formation of the capsule.116,117 In particular, as shown in Scheme 10, the inner space of the capsule mainly leads to the formation of the trans pseudo-photostationary state of the stilbenes, which precludes the cyclization into the final product. The general ability of the capsule to control photo-dimerization reactions has been also studied with methyl cinnamates, p-methyl styrene, indene, and 4,4-dimethylcyclohex-2-enone.118
2 stoichiometry from the gas phase and the aqueous phase.108 Due to the high difference of binding constant values between propane and butane, capsule 7 can selectively extract butane from a propane/butane mixture. Ramamurthy and co-workers used the OA capsule 7 as chemical reactor in aqueous solution,109 for the generation of singlet oxygen,110,111 the photodimerization of acenaphthylene,112 and the photo-induced rearrangement of dibenzylketone.109,113 Furthermore, this nano-capsule can affect the photochemistry of α-(n-alkyl) dibenzyl ketones depending on the dimension of the guests:114 different kinds of packing motif lead to diverse photochemical behaviors. With the smallest guests a simple decarbonylation is observed, whereas, rearrangement products became prominent with the guests that possess bulkier alkyl groups. To clarify the mechanism of this rearrangement, optically pure α-deoxybenzoins were used as guests contained into OA capsule.115 In addition, analysis with stilbene derivatives as substrate demonstrated that photophysical and photochemical properties of the encapsulated guests are strongly influenced by the formation of the capsule.116,117 In particular, as shown in Scheme 10, the inner space of the capsule mainly leads to the formation of the trans pseudo-photostationary state of the stilbenes, which precludes the cyclization into the final product. The general ability of the capsule to control photo-dimerization reactions has been also studied with methyl cinnamates, p-methyl styrene, indene, and 4,4-dimethylcyclohex-2-enone.118
Ramamurthy reported on another example of how the inner space of octa acid capsule alters the outcome of some photochemical reactions. By photochemical reaction, cyclohexyl-phenyl ketones can afford Type I and Type II Norrish products. Cyclobutanol, the Type II Norrish product, is formed in free solution of acetonitrile, while only the Type I products are obtained with octa acid capsule (Scheme 11).119
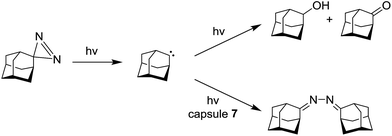
The inner space of octa acid container 7 can also influence the chemical behavior of incarcerated carbenes generated by photolysis of adamantanediazirines.120
In free solution, formation of alcohol and ketone was observed, while carbene generated into OA capsule furnished the azine derivative in high yield (Scheme 12).
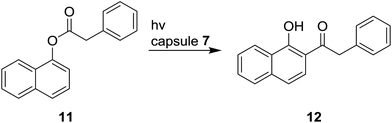
Gibb has also reported that OA 7 controls the product distribution during photo-Fries rearrangement of naphthyl esters in water by restricting the mobility of primary singlet radical pair.121 In normal condition, ester 11 in hexane upon irradiation affords eight products, while, after inclusion into the cavity of 7, only compound 12 is formed in 99% yield (Scheme 13).
The same ortho-rearrangement was observed with other ester compounds. The high selectivity was attributed to the limited movement of the guest inside the capsular assembly, and the rapid recombination of the radicals.
Gibb's group described the kinetic resolution of pairs of constitutional isomers of long chain esters using the octa acid capsule 7, that cannot be resolved since they react at similar rates.122 Their strategy was based on the higher affinity of one guest to be preferentially encapsulated into the capsule 7, leaving the other molecule to undergo hydrolysis in solution. The resolution is determined by a competitive binding equilibrium in which the stronger binder mainly occupies the inner space of the capsule, whereas the weaker binding ester mostly resides in the bulk hydrolytic medium.
Supramolecular cage 7 has been used to carry out diastereoselective photoreactions. In particular, photocyclization of tropolone ethers and the oxa-di-π-methane rearrangement of cyclohexadienones within the capsule revealed good diastereoselectivities inside the capsule (17%) in comparison with the bulk solution (3%) (Scheme 14).123
A higher enhancement of diastereoselectivity inside the capsule 7 (73%) with respect to the free solution (4%) was obtained by Gibb and co-workers, while studying the photoelectrocyclization of pyridones.124
Recent studies highlighted how these water soluble capsules can act as active species in some photochemical reactions.125 In fact, combining octa acid cavitand 7 with a resorcinol-capped octa acid cavitand (devoid of carboxylic groups at the upper rim and with four resorcinol groups at the lower rim), a hetero-capsule was formed and able to act as triplet sensitizers towards aromatic guests, leading to different products depending on the type of excitation (Scheme 15).
Recently, Ramamurthy emphasized how the guests inside OA 7 capsule undergo a pre-orientation effect, because all parts of the enclosed guest molecules are exposed only to a hydrophobic inner space, thus excluding any orientation from hydrophobic–hydrophilic interactions, characteristics of other opened molecular reactors. This feature is particularly important in the regio- and stereo-selectivity of the reaction catalyzed inside the capsule.126 In the photodimerization of indene in acetonitrile (Scheme 16), four possible reaction products are possible. However, in the presence of OA 7, anti-head–tail dimer is exclusively formed inside the cavity.
An interesting application of capsule 7 was recently reported by Ramamurthy's research group. They explored the feasibility of opening the capsule in order to release its contents in an aqueous media.127 In particular, p-methoxyphenacyl esters were photo-hydrolized in ca. 90 minutes leading to the corresponding acid derivatives as main products, and the disassembly of the capsule, hence releasing the products in solution. This system presents potential applications in the selective photo-release of molecules for pharmaceutical purposes.
Ramamurthy's group have explored the properties of the OA 7 as photo-reactor on the silica surface.128 They demonstrated that the properties of photo-reactor are maintained in the heterogeneous system, and that capsular assemblies are stable on silica surface.
Very recently, Ramamurthy and co-workers reported on the photorelease of different carboxylic acids hosted into the OA capsule 7, after a rapid photo-Favorskii rearrangement of p-hydroxyphenacyl esters (Scheme 17).129
 | ||
| Scheme 18 Resorcin[4]arene 13 and relative hexameric capsule 14. Adapted with permission from J. Am. Chem. Soc., 2008, 130, 2344–2350. Copyright 2008 American Chemical Society. | ||
In particular, the acids were esterified with photoremovable protecting groups (PPGs), which upon irradiation >300 nm for 50 minutes undergo to the hydrolysis of the ester bonds. The absence of radical attack on the OA capsule 7 by p-hydroxyphenacyl ester photolysis is a clear evidence that the photorelease mechanism for OA encapsulated p-hydroxyphenacyl esters differs from that of the p-methoxyphenacyl esters.
Hexameric supramolecular capsules
By the formation of multiple hydrogen bonds, in apolar solvents, the resorcin[4]arene 13 can self-assembly to the hexameric capsule 14 (Scheme 18), which was reported for the first time by Atwood in 1997.130,131With an internal volume of ca. 1400 Å3, hexameric capsule 14 represents one of the largest hydrogen-bonded molecular capsules. The presence of eight water molecules is essential to the formation of this assembly.132 The formation of the capsule and the encapsulation of different guests have been extensively investigated in solution.133–142
Tiefenbacher and Zhang reported on the first catalytic application of capsule 14: due to the value of pKa (approximately 5.5–6), the capsule acts as a strong Brønsted acid.143 The authors explored the possibility to protonate a stabilized Wittig ylide inside the capsule 14 (Scheme 19). Ylide 15 is included inside the hexameric capsule 14, as confirmed by NMR spectroscopy. Even the addition of an excess of propanal (10 equiv.) did not produce any alkene product, although the uptake of propanal into the capsule is demonstrated by the appearance of characteristic signals in the NMR spectra.
With the larger Wittig ester 16, which is not able to fit the inner space of the capsule, the formation of alkene 17 occurred, indicating that the protonation of the Wittig ylide outside the capsule is reversible and does not prevent conversion to the alkene. Furthermore, the authors explored the potential use of the hexameric capsule as a selective enzyme-like catalyst, investigating the hydrolysis of acetals. They proved that a catalytic conversion is indeed possible with the capsule and that the reaction takes place inside the cavity. In addition, comparing the results obtained by using different substrates, they concluded that hexameric capsule 14, due to the stabilization of cationic intermediates and transition states in the hydrolysis process, enables reactions under mild conditions that are not possible in the solution phase.
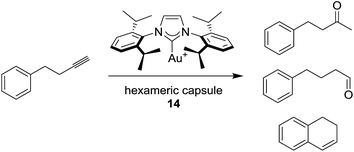
Reek and co-workers used hexameric capsule 14 to include gold complexes, obtaining a supramolecular catalyst for the hydration of 4-phenyl-butyne (Scheme 20).144
This reaction normally occurs with Markovnikov addition of water, leading to the corresponding ketone, or 1,2 dihydronaphtalene in absence of water. In the presence of hexameric capsule 14 the formation of aldehyde is observed, as well as the ketone and 1,2 dihydronaphtalene with different ratio respect to the normal reaction in solution. Afterward, the same research group evaluated the impact of steric hindrance and structural rigidity of the guests on the rate of reaction.145 Interestingly, the reaction rates of the Au-catalyst in absence of the hexameric capsule show an opposite trend respect to the supramolecular catalyst.
Very recently, Scarso and co-workers reported on the use of 14 as a nanoreactor in the synthesis of several amide derivatives, differing for the length of substituent R1 and R2 (Scheme 21).146
The capsule acts as nanoreactor that imparts steric restriction to the encapsulated reagent. In particular, the capsule includes the cationic carbodiimide activator, and preferentially select the shorter acidic and amine reagents leading to the shorter amide derivatives. In absence of the supramolecular cage, scarce product selectivities were detected, thus demonstrating the active role of the hexameric capsule.
AFM applications
AFM (Atomic Force Microscopy) is a powerful technique, applied mainly to the material science, with several applications in imaging and manipulation of the surfaces at nanoscale level.147,148 The basic principle is based on a small force sensor (cantilever) and a piezo scan tube positioned onto the sample. The sharp tip of the cantilever, which has a radius of ca. 10 nm, interacts with the substrate via a wide range of weak forces, and this phenomenon leads to a minimal deflection of the sensor (Fig. 5). Furthermore, nano-scale objects can be precisely manipulated with the cantilever tip by applying very low forces (in the range of pN = 10–12 N), which is the magnitude of weak intermolecular bonds. A typical AFM-SMFS (AFM-single molecule force spectroscopy) mechano-chemistry experiment consists in the monitoring of interactions between two molecules, i.e. a host and a guest, using this technique. The tip of the cantilever is functionalized with a flexible linker (typically a PEG spacer) bearing one of the molecules object of the study. At the same time, the surface is covered by the other “partner” molecule.149–151A precise determination of the forces between host and guest occurs, following the deflection on the cantilever. For this reason, the development of new synthetic receptors able to exploit non-covalent interactions using different weak forces is of primary importance in this technique. The first problem to solve is the covalent anchoring of host.
One of the most common strategies is the functionalization of the lower rim with sulfur-containing groups for its attachment onto a gold surface.152,153 For SMFS experiments, Schindler's research group immobilized a tetra-(carboxyl)cavitand with a PEG linker to the cantilever, to add steric freedom to the system and allowing the complex formation to take place (Fig. 6).154
The spacer was further modified introducing a new type of linker via “click chemistry”.155 The first set of experiments were carried out using a tetra-(pyridyl)cavitand covalently attached on the gold surface, and a tetra-(carboxyl)cavitand immobilized on the cantilever. All measurements were carried out in p-xylene, which is encapsulated during the assembly process. Because of the high concentration of the tetra-(pyridyl)cavitand in the surface, multiple interactions due to simultaneous formation of several supramolecular capsules were detected.
Moreover, mixed self-assembled monolayers (SAMs) containing alkyl sulfides without functional groups can be used to realize a functionalized surface at low density, thus ensuring single-molecule recognition.156,157 The force–distance curve observed contains only one distinct force jump, associated to the dissociation of a supramolecular capsule formed between the two hosts and p-xylene. Plotting the detected dissociation forces in a force histogram, a narrow distribution of forces characteristic for single-molecule force spectroscopy experiments was observed, allowing for a precise determination of the dissociation force, along with the mechanical stability and the dynamics of the dissociation process of this supramolecular capsule in a quantitative manner.
Recently, AFM measurements were employed to quantitatively evaluate drug–receptor interactions,158 and drug–cell interactions159 for analytical applications. Furthermore, these studies had lead to the development of new drugs with higher efficiency and also give fundamental insight into the mechanism of molecular level interactions in biological systems.
Conclusion and outlook
In this review we detailed supramolecular capsules based on resorcin[4]arenes, calix[n]arenes and metal–ligands, having concrete applications in biomedical field, catalysis and material science. The inner space of these self-assembled structures represents a unique environment, with chemical–physical properties different with respect to the bulk solution. For this reason, one (or more) guest molecule encapsulated in this nanospace can undergo a different “chemistry” relative to the normal conditions. This feature is of particular interest in catalysis, leading to nanoreactors able to catalyze reactions not allowed in normal solution, or with the classical organo-metallic catalysts, or in biomedical applications, obtaining efficient drug-delivery system with potential theranostic properties. Although these supramolecular systems are known since '80, the research interest to design new and efficient (supra)molecular architectures, with practical uses in medicinal and materials, is still today an important and active field.Acknowledgements
We thank University of Catania for the financial support.Notes and references
- T. Ogoshi and T. Yamagishi, Pillararenes: versatile synthetic receptors for supramolecular chemistry, Eur. J. Org. Chem., 2013, 2961–2975 CrossRef CAS PubMed.
- J.-M. Lehn, Perspectives in chemistry—aspects of adaptive chemistry and materials, Angew. Chem., Int. Ed., 2015, 54, 3276–3289 CrossRef CAS PubMed.
- L. F. Lindoy, K.-M. Park and S. S. Lee, Metals, macrocycles and molecular assemblies – macrocyclic complexes in metallo-supramolecular chemistry, Chem. Soc. Rev., 2013, 42, 1713–1727 RSC.
- K. Kirkorian, A. Ellis and L. J. Twyman, Catalytic hyperbranched polymers as enzyme mimics; exploiting the principles of encapsulation and supramolecular chemistry, Chem. Soc. Rev., 2012, 41, 6138–6159 RSC.
- Supramolecular Chemistry: From Molecules to Nanomaterials, ed. P. Gale and J. Steed, Wiley-VCH, Weinheim, 2012 Search PubMed.
- D. M. Bassani, Supramolecular chemistry: Molecular wires get connected, Nature, 2011, 480, 326–327 CrossRef CAS PubMed.
- L. A. Joyce, S. H. Shabbir and E. V. Anslyn, The uses of supramolecular chemistry in synthetic methodology development: examples of anion and neutral molecular recognition, Chem. Soc. Rev., 2010, 39, 3621–3632 RSC.
- S. I. Stupp and L. C. Palmer, Supramolecular chemistry and self-assembly in organic materials design, Chem. Mater., 2014, 26, 507–518 CrossRef CAS.
- R. C. Elgersma, T. Meijneke, R. de Jong, A. J. Brouwer, G. Posthuma, D. T. S. Rijkersa and R. M. J. Liskamp, Synthesis and structural investigations of N-alkylated β-peptidosulfonamide–peptide hybrids of the amyloidogenic amylin(20–29) sequence: implications of supramolecular folding for the design of peptide-based bionanomaterials, Org. Biomol. Chem., 2006, 4, 3587–3597 CAS.
- E. Persch, O. Dumele and F. Diederich, Molecular recognition in chemical and biological systems, Angew. Chem., Int. Ed., 2015, 54, 3290–3327 CrossRef CAS PubMed.
- C. J. Bruns and J. F. Stoddart, Rotaxane-based molecular muscles, Acc. Chem. Res., 2014, 47, 2186–2199 CrossRef CAS PubMed.
- R. Dong, Y. Zhou, X. Huang, X. X. Zhu, Y. Lu and J. Shen, Functional supramolecular polymers for biomedical applications, Adv. Mater., 2015, 27, 498–526 CrossRef CAS PubMed.
- R. B. K. Siram, M. Stephen, F. Ali and S. Patil, Investigation of phase separation in bulk heterojunction solar cells via supramolecular chemistry, J. Phys. Chem. C, 2013, 117, 9129–9136 CAS.
- C. Bazzicalupi, A. Bianchi, E. Garcia-Espana and E. Delgado-Pinar, Metals in supramolecular chemistry, Inorg. Chim. Acta, 2014, 417, 3–26 CrossRef CAS PubMed.
- D. J. Cram, S. Karbach, Y. H. Kim, L. Baczynskyj and G. W. Kalleymeyn, Shell closure of two cavitands forms carcerand complexes with components of the medium as permanent guests, J. Am. Chem. Soc., 1985, 107, 2575–2576 CrossRef CAS.
- R. Wyler, J. de Mendoza and J. Rebek Jr, A synthetic cavity assembles through self-complementary hydrogen bonds, Angew. Chem., Int. Ed. Engl., 1993, 32, 1699–1701 CrossRef PubMed.
- M. Fujita, M. Tominaga, A. Hori and B. Therrien, Coordination assemblies from a Pd(II)-cornered square complex, Acc. Chem. Res., 2005, 38, 369–378 CrossRef CAS PubMed.
- M. Yoshizawa, J. K. Klosterman and M. Fujita, Functional molecular flasks: new properties and reactions within discrete, self-assembled hosts, Angew. Chem., Int. Ed., 2009, 48, 3418–3438 CrossRef CAS PubMed.
- B. H. Northrop, Y.-R. Zheng, K.-W. Chi and P. J. Stang, Self-organization in coordination-driven self-assembly, Acc. Chem. Res., 2009, 42, 1554–1563 CrossRef CAS PubMed.
- J. Rebek Jr, Molecular behavior in small spaces, Acc. Chem. Res., 2009, 42, 1660–1668 CrossRef PubMed.
- B. Breiner, J. K. Clegg and J. R. Nitschke, Reactivity modulation in container molecules, Chem. Sci., 2011, 2, 51–56 RSC.
- O. Mayzel and Y. Cohen, Diffusion coefficients of macrocyclic complexes using the PGSE NMR technique: determination of association constants, J. Chem. Soc., Chem. Commun., 1994, 1901–1902 RSC.
- O. Mayzel, O. Aleksiuk, F. Grynszpan, S. E. Biali and Y. Cohen, NMR diffusion coefficients of p-tert-butylcalix[n]arene systems, J. Chem. Soc., Chem. Commun., 1995, 1183–1184 RSC.
- O. Mayzel, A. Gafni and Y. Cohen, Water hydration of macrocyclic systems in organic solvents: an NMR diffusion and chemical shift study, Chem. Commun., 1996, 911–912 RSC.
- A. Gafni and Y. Cohen, Complexes of macrocycles with γ-cyclodextrin as deduced from NMR diffusion measurements, J. Org. Chem., 1997, 62, 120–125 CrossRef CAS PubMed.
- T. Parella, High-quality 1D spectra by implementing pulsed-field gradients as the coherence pathway selection procedure, Magn. Reson. Chem., 1996, 34, 329–347 CrossRef CAS.
- T. Parella, Pulsed field gradients: a new tool for routine NMR, Magn. Reson. Chem., 1998, 36, 467–495 CrossRef CAS.
- C. S. Johnson Jr, Diffusion ordered nuclear magnetic resonance spectroscopy: principles and applications, Prog. Nucl. Magn. Reson. Spectrosc., 1999, 34, 203–256 CrossRef.
- A. Gafni, Y. Cohen, S. Palmer and D. Parker, Enantiomer discrimination using lipophilic cyclodextrins studied by electrode response, pulsed-gradient spin-echo (PGSE) NMR and relaxation rate measurements, J. Chem. Soc., Perkin Trans. 2, 1998, 19–24 RSC.
- P. Timmerman, J. L. Weidmann, K. A. Jolliffe, L. J. Prins, D. N. Reinhoudt, S. Shinkai, L. Frish and Y. Cohen, NMR diffusion spectroscopy for the characterization of multicomponent hydrogen-bonded assemblies in solution, J. Chem. Soc., Perkin Trans. 2, 2000, 2077–2089 RSC.
- L. Avram and Y. Cohen, Complexation in pseudorotaxanes based on α-cyclodextrin and different α,ω-diaminoalkanes by NMR diffusion measurements, J. Org. Chem., 2002, 67, 2639–2644 CrossRef CAS PubMed.
- L. Frish, F. Sansone, A. Casnati, R. Ungaro and Y. Cohen, Complexation of a peptidocalix[4]arene, a vancomycin mimic, with alanine-containing guests by NMR diffusion measurements, J. Org. Chem., 2000, 65, 5026–5030 CrossRef CAS.
- K. S. Cameron and L. Fielding, NMR diffusion spectroscopy as a measure of host−guest complex association constants and as a probe of complex size, J. Org. Chem., 2001, 66, 6891–6895 CrossRef CAS PubMed.
- L. Fielding, NMR methods for the determination of protein-ligand dissociation constants, Curr. Top. Med. Chem., 2003, 3, 39–53 CrossRef CAS.
- A. Pappalardo, M. E. Amato, F. P. Ballistreri, G. A. Tomaselli, R. M. Toscano and G. Trusso Sfrazzetto, Pair of diastereomeric uranyl salen cavitands displaying opposite enantiodiscrimination of α-amino acid ammonium salts, J. Org. Chem., 2012, 77, 7684–7687 CrossRef CAS PubMed.
- F. P. Ballistreri, A. Pappalardo, R. M. Toscano, G. A. Tomaselli and G. Trusso Sfrazzetto, Heteroditopic chiral uranyl–salen receptor for molecular recognition of amino acid ammonium salts, Eur. J. Org. Chem., 2010, 3806–3810 CrossRef CAS PubMed.
- M. E. Amato, F. P. Ballistreri, S. D'Agata, A. Pappalardo, G. A. Tomaselli, R. M. Toscano and G. Trusso Sfrazzetto, Enantioselective molecular recognition of chiral organic ammonium ions and amino acids using cavitand–salen-based receptors, Eur. J. Org. Chem., 2011, 5674–5680 CrossRef CAS PubMed.
- A. Pappalardo, M. E. Amato, F. P. Ballistreri, A. Notti, G. A. Tomaselli, R. M. Toscano and G. Trusso Sfrazzetto, Synthesis and topology of [2 + 2] calix[4]resorcarene-based chiral cavitand-salen macrocycles, Tetrahedron Lett., 2012, 53, 7150–7153 CrossRef CAS PubMed.
- M. L. Giuffrida, E. Rizzarelli, G. A. Tomaselli, C. Satriano and G. Trusso Sfrazzetto, A novel fully water-soluble Cu(I) probe for fluorescence live cell imaging, Chem. Commun., 2014, 9835–9838 RSC.
- P. S. Pregosin, P. G. A. Kumar and I. Fernandez, Pulsed gradient spin−echo (PGSE) diffusion and 1H,19F heteronuclear overhauser spectroscopy
(HOESY) NMR methods in inorganic and organometallic chemistry:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) something old and something new, Chem. Rev., 2005, 105, 2977–2998 CrossRef CAS PubMed.
something old and something new, Chem. Rev., 2005, 105, 2977–2998 CrossRef CAS PubMed. - S. V. Kharlamov and S. V. Latypov, Modern diffusion-ordered NMR spectroscopy in chemistry of supramolecular systems: the scope and limitations, Russ. Chem. Rev., 2010, 79, 635–653 CrossRef CAS PubMed.
- A. Macchioni, G. Ciancaleoni, C. Zuccaccia and D. Zuccaccia, Determining accurate molecular sizes in solution through NMR diffusion spectroscopy, Chem. Soc. Rev., 2008, 37, 479–489 RSC.
- G. M. Lombardo, A. L. Thompson, F. P. Ballistreri, A. Pappalardo, G. Trusso Sfrazzetto, G. A. Tomaselli, R. M. Toscano and F. Punzo, An integrated X-ray and molecular dynamics study of uranyl-salen structures and properties, Dalton Trans., 2012, 41, 1951–1960 RSC.
- G. Brancatelli, A. Pappalardo, G. Trusso Sfrazzetto, A. Notti and S. Geremia, Mono- and dinuclear uranyl(VI) complexes with chiral Schiff base ligand, Inorg. Chim. Acta, 2013, 396, 25–29 CrossRef CAS PubMed.
- L. Allouche, A. Marquis and J.-M. Lehn, Discrimination of metallosupramolecular architectures in solution by using diffusion ordered spectroscopy (DOSY) experiments: double-stranded helicates of different lengths, Chem.–Eur. J., 2006, 12, 7520–7525 CrossRef CAS PubMed.
- N. Giuseppone, J.-L. Schmitt, L. Allouche and J.-M. Lehn, DOSY NMR experiments as a tool for the analysis of constitutional and motional dynamic processes: implementation for the driven evolution of dynamic combinatorial libraries of helical strands, Angew. Chem., Int. Ed., 2008, 47, 2235–2239 CrossRef CAS PubMed.
- F. Gulion, R. Lauceri, L. Frish, T. Evan-Salem, Y. Cohen, R. De Zorzi, S. Geremia, L. Di Costanzo, L. Randaccio, D. Sciotto and R. Purrello, Noncovalent synthesis in aqueous solution and spectroscopic characterization of multi-porphyrin complexes, Chem.–Eur. J., 2006, 12, 2722–2729 CrossRef PubMed.
- T. Evan-Salem, L. Frish, F. W. B. van Leeuwen, D. N. Reinhoudt, W. Verboom, M. S. Kaucher, J. T. Davis and Y. Cohen, Self-assembled ionophores from isoguanosine: diffusion NMR spectroscopy clarifies cation's and anion's influence on supramolecular structure, Chem.–Eur. J., 2007, 13, 1969–1977 CrossRef CAS PubMed.
- G. Gattuso, A. Notti, A. Pappalardo, S. Pappalardo and M. F. Parisi, A supramolecular amphiphile from a new water-soluble calix[5]arene and n-dodecylammonium chloride, Tetrahedron Lett., 2013, 54, 188–191 CrossRef CAS PubMed.
- S. Pappalardo, V. Villari, S. Slovak, Y. Cohen, G. Gattuso, A. Notti, A. Pappalardo, I. Pisagatti and M. F. Parisi, Counterion-dependent proton-driven self-assembly of linear supramolecular oligomers based on amino-calix[5]arene building blocks, Chem.–Eur. J., 2007, 13, 8164–8173 CrossRef CAS PubMed.
- J. M. Zayed, F. Biedermann, U. Rauwald and O. A. Scherman, Probing cucurbit[8]uril-mediated supramolecular block copolymer assembly in water using diffusion NMR, Polym. Chem., 2010, 1, 1434–1436 RSC.
- Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Formation of linear supramolecular polymers that is driven by C–H⋯π interactions in solution and in the solid state, Angew. Chem., Int. Ed., 2011, 50, 1397–1401 CrossRef CAS PubMed.
- A. Pappalardo, F. P. Ballistreri, G. Li Destri, P. G. Mineo, G. A. Tomaselli, R. M. Toscano and G. Trusso Sfrazzetto, Supramolecular polymer networks based on calix[5]arene tethered poly(p-phenyleneethynylene), Macromolecules, 2012, 45, 7549–7556 CrossRef CAS.
- L. Avram and Y. Cohen, Diffusion NMR of molecular cages and capsules, Chem. Soc. Rev., 2015, 44, 586–602 RSC.
- J. Braun, K. Renggli, J. Razumovitch and C. Vebert in Dynamic Light Scattering in Supramolecular Materials Chemistry, Supramolecular Chemistry: From Molecules to Nanomaterials, ed. P. Gale and J. Steed, Wiley-VCH, Weinheim, 2012 Search PubMed.
- D.-S. Gu and Y. Liu, Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications, Acc. Chem. Res., 2014, 47, 1925–1934 CrossRef PubMed.
- F. Perret, A. N. Lazar and A. W. Coleman, Biochemistry of the para-sulfonato-calix[n]arenes, Chem. Commun., 2006, 2425–2438 RSC.
- A. Arduini, A. Pochini, S. Raverberi and R. Ungaro, p-t-Butyl-calix[4]arene tetracarboxylic acid. A water soluble calixarene in a cone structure, J. Chem. Soc., Chem. Commun., 1984, 981–982 RSC.
- P. Shahgaldian, A. W. Coleman and V. I. Kalchenko, NMR-studies on sugars and cyclanols - III on the configuration of C-methyl-branched sugars and cyclanols at the branching point, Tetrahedron Lett., 2001, 42, 577–582 CrossRef CAS.
- E.-H. Ryu and Y. Zhao, Efficient synthesis of water-soluble calixarenes using click chemistry, Org. Lett., 2005, 7, 1035–1037 CrossRef CAS PubMed.
- A. W. Coleman, S. Jebors, S. Cecillon, P. Perret, D. Garin, D. Marti-Battle and M. Moulin, Toxicity and biodistribution of para-sulfonato-calix[4]arene in mice, New J. Chem., 2008, 32, 780–782 RSC.
- K. Yannakopoulou, Cationic cyclodextrins: cell penetrating agents and other diverse applications, J. Drug Delivery Sci. Technol., 2012, 22, 243–249 CrossRef CAS.
- I. Ghosh and W. M. Nau, The strategic use of supramolecular pK(a) shifts to enhance the bioavailability of drugs, Adv. Drug Delivery Rev., 2012, 64, 764–783 CrossRef CAS PubMed.
- C. J. Porter, N. L. Trevaskis and W. N. Charman, Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs, Nat. Rev. Drug Discovery, 2007, 6, 231–248 CrossRef CAS PubMed.
- C. A. Lipinski, Drug-like properties and the causes of poor solubility and poor permeability, J. Pharmacol. Toxicol. Methods, 2000, 44, 235–249 CrossRef CAS.
- R. Haag, Supramolecular drug-delivery systems based on polymeric core–shell architectures, Angew. Chem., Int. Ed., 2004, 43, 278–282 CrossRef CAS PubMed.
- F. Hirayama and K. Uekama, Cyclodextrin-based controlled drug release system, Adv. Drug Delivery Rev., 1999, 36, 125–141 CrossRef CAS.
- S. Menon, N. Modi, B. Mistry and K. Joshi, Improvement of some pharmaceutical properties of mycophenolate mofetil (MMF) by para sulphonatocalix[4]resorcinarene inclusion complex, J. Inclusion Phenom. Mol. Recognit. Chem., 2011, 70, 121–128 CrossRef CAS.
- S. Menon, B. Mistry, K. Joshi, N. Modi and D. Shashtri, Evaluation and solubility improvement of Carvedilol: PSC[n]arene inclusion complexes with acute oral toxicity studies, J. Inclusion Phenom. Mol. Recognit. Chem., 2012, 73, 295–303 CrossRef CAS PubMed.
- M. B. Patel, N. N. Valand, N. R. Modi, K. V. Joshi, U. Harikrishnan, S. P. Kumar, Y. T. Jasrai and S. Menon, Effect of p-sulfonatocalix[4]resorcinarene (PSC[4]R) on the solubility and bioavailability of a poorly water soluble drug lamotrigine (LMN) and computational investigation, RSC Adv., 2013, 3, 15971–15981 RSC.
- Y. Xue, Y. Guan, A. Zheng and H. Xiao, Amphoteric calix[8]arene-based complex for pH-triggered drug delivery, Colloids Surf., B, 2013, 101, 55–60 CrossRef CAS PubMed.
- Y.-R. Zheng, K. Suntharalingam, T. C. Johnstone and S. J. Lippard, Encapsulation of Pt(IV) prodrugs within a Pt(II) cage for drug delivery, Chem. Sci., 2015, 6, 1189–1193 RSC.
- M. Fujita, D. Oguro, M. Mlyazawa, H. Oka, K. Yamaguchi and K. Ogura, Self-assembly of ten molecules into nanometre-sized organic host frameworks, Nature, 1995, 378, 469–471 CrossRef CAS PubMed.
- S. Tashiro, M. Tominaga, M. Kawano, B. Therrien, T. Ozekiand and K. Fujita, Sequence-selective recognition of peptides within the single binding pocket of a self-assembled coordination cage, J. Am. Chem. Soc., 2005, 127, 4546–4547 CrossRef CAS PubMed.
- K. Nakabayashi, M. Kawano and M. Fujita, pH-Switchable through-space interaction of organic radicals within a self-assembled coordination cage, Angew. Chem., Int. Ed., 2005, 44, 5322–5325 CrossRef CAS PubMed.
- K. Nakabayashi, M. Kawano, T. Kato, K. Furukawa, S. I. Ohkoshi, T. Hozumi and M. Fujita, Manipulating the through-space spin–spin interaction of organic radicals in the confined cavity of a self-assembled cage, Chem.–Asian J., 2007, 2, 164–170 CrossRef CAS PubMed.
- K. Nakabayashi, Y. Ozaki, M. Kawano and M. Fujita, A self-assembled spin cage, Angew. Chem., Int. Ed., 2008, 47, 2046–2048 CrossRef CAS PubMed.
- Y. Nishioka, T. Yamaguchi, M. Kawano and M. Fujita, Asymmetric [2 + 2] olefin cross photoaddition in a self-assembled host with remote chiral auxiliaries, J. Am. Chem. Soc., 2008, 130, 8160–8161 CrossRef CAS PubMed.
- Y. Nishioka, T. Yamaguchi, M. Yoshizawa and M. Fujita, Unusual [2+4] and [2+2] cycloadditions of arenes in the confined cavity of self-assembled cages, J. Am. Chem. Soc., 2007, 129, 7000–7001 CrossRef CAS PubMed.
- T. Yamaguchi and M. Fujita, Highly selective photomediated 1,4-radical addition to o-quinones controlled by a self-assembled cage, Angew. Chem., Int. Ed., 2008, 47, 2067–2069 CrossRef CAS PubMed.
- S. Karthikeyan and V. Ramamurthy, Templating photodimerization of trans-cinnamic acid esters with a water-soluble Pd nanocage, J. Org. Chem., 2007, 72, 452–458 CrossRef CAS PubMed.
- S. Karthikeyan and V. Ramamurthy, Templating Photodimerization of Coumarins within a Water-Soluble Nano Reaction Vessel, J. Org. Chem., 2006, 71, 6409–6413 CrossRef CAS PubMed.
- S. Karthikeyan and V. Ramamurthy, Self-assembled coordination cage as a reaction vessel: triplet sensitized [2+2] photodimerization of acenaphthylene, and [4+4] photodimerization of 9-anthraldehyde, Tetrahedron Lett., 2005, 46, 4495–4498 CrossRef CAS PubMed.
- A. V. Davis, D. Fiedler, F. E. Ziegler, A. Terpin and K. N. Raymond, Resolution of chiral, tetrahedral M4L6 metal−ligand hosts, J. Am. Chem. Soc., 2007, 129, 15354–15363 CrossRef CAS PubMed.
- S. M. Biros, R. G. Bergman and K. N. Raymond, The hydrophobic effect drives the recognition of hydrocarbons by an anionic metal−ligand cluster, J. Am. Chem. Soc., 2007, 129, 12094–12095 CrossRef CAS PubMed.
- C. J. Hastings, M. D. Pluth, S. M. Biros, R. G. Bergman and K. N. Raymond, Simultaneously bound guests and chiral recognition: a chiral self-assembled supramolecular host encapsulates hydrophobic guests, Tetrahedron, 2008, 64, 8362–8367 CrossRef CAS PubMed.
- V. M. Dong, D. Fiedler, B. Carl, R. G. Bergman and K. N. Raymond, Molecular recognition and stabilization of iminium ions in water, J. Am. Chem. Soc., 2006, 128, 14464–14465 CrossRef CAS PubMed.
- D. Fiedler, R. G. Bergman and K. N. Raymond, Stabilization of reactive organometallic intermediates inside a self-assembled nanoscale host, Angew. Chem., Int. Ed., 2006, 45, 745–748 CrossRef CAS PubMed.
- D. H. Leung, R. G. Bergman and K. N. Raymond, Scope and mechanism of the C−H bond activation reactivity within a supramolecular host by an iridium guest:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a stepwise ion pair guest dissociation mechanism, J. Am. Chem. Soc., 2006, 128, 9781–9797 CrossRef CAS PubMed.
a stepwise ion pair guest dissociation mechanism, J. Am. Chem. Soc., 2006, 128, 9781–9797 CrossRef CAS PubMed. - D. H. Leung, R. G. Bergman and K. N. Raymond, Highly selective supramolecular catalyzed allylic alcohol isomerization, J. Am. Chem. Soc., 2007, 129, 2746–2747 CrossRef CAS PubMed.
- C. J. Brown, G. M. Miller, M. W. Johnson, R. G. Bergman and K. N. Raymond, High-turnover supramolecular catalysis by a protected ruthenium(II) complex in aqueous solution, J. Am. Chem. Soc., 2011, 133, 11964–11966 CrossRef CAS PubMed.
- Z. J. Wang, K. N. Clary, R. G. Bergman, K. N. Raymond and F. D. Toste, A supramolecular approach to combining enzymatic and transition metal catalysis, Nat. Chem., 2013, 5, 100–105 CrossRef CAS PubMed.
- Z. J. Wang, C. J. Brown, R. G. Bergman, K. N. Raymond and F. D. Toste, Hydroalkoxylation catalyzed by a gold(I) complex encapsulated in a supramolecular host, J. Am. Chem. Soc., 2011, 133, 7358–7360 CrossRef CAS PubMed.
- W. M. Hart-Cooper, K. N. Clary, F. D. Toste, R. G. Bergman and K. N. Raymond, Selective monoterpene-like cyclization reactions achieved by water exclusion from reactive intermediates in a supramolecular catalyst, J. Am. Chem. Soc., 2012, 134, 17873–17876 CrossRef CAS PubMed.
- D. Fiedler, H. van Halbeek, R. G. Bergman and K. N. Raymond, Supramolecular catalysis of unimolecular rearrangements:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate scope and mechanistic insights, J. Am. Chem. Soc., 2006, 128, 10240–10252 CrossRef CAS PubMed.
substrate scope and mechanistic insights, J. Am. Chem. Soc., 2006, 128, 10240–10252 CrossRef CAS PubMed. - C. J. Hastings, D. Fiedler, R. G. Bergman and K. N. Raymond, Aza cope rearrangement of propargyl enammonium cations catalyzed by a self-assembled “nanozyme”, J. Am. Chem. Soc., 2008, 130, 10977–10983 CrossRef CAS PubMed.
- M. D. Pluth, R. G. Bergman and K. N. Raymond, Making amines strong bases:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thermodynamic stabilization of protonated guests in a highly-charged supramolecular host, J. Am. Chem. Soc., 2007, 129, 11459–11467 CrossRef CAS PubMed.
thermodynamic stabilization of protonated guests in a highly-charged supramolecular host, J. Am. Chem. Soc., 2007, 129, 11459–11467 CrossRef CAS PubMed. - M. D. Pluth, R. G. Bergman and K. N. Raymond, Acid catalysis in basic solution: a supramolecular host promotes orthoformate hydrolysis, Science, 2007, 316, 85–88 CrossRef CAS PubMed.
- M. D. Pluth, R. G. Bergman and K. N. Raymond, Catalytic deprotection of acetals in basic solution with a self-assembled supramolecular “nanozyme”, Angew. Chem., Int. Ed., 2007, 46, 8587–8589 CrossRef CAS PubMed.
- M. D. Pluth, R. G. Bergman and K. N. Raymond, The acid hydrolysis mechanism of acetals catalyzed by a supramolecular assembly in basic solution, J. Org. Chem., 2009, 74, 58–63 CrossRef CAS PubMed.
- S. M. Biros and J. Rebek Jr, Structure and binding properties of water-soluble cavitands and capsules, Chem. Soc. Rev., 2007, 36, 93–104 RSC.
- G. V. Oshovsky, D. N. Reinhoudt and W. Verboom, Supramolecular chemistry in water, Angew. Chem., Int. Ed., 2007, 46, 2366–2393 CrossRef CAS PubMed.
- C. L. D. Gibb and B. C. Gibb, Well-defined, organic nanoenvironments in water: the hydrophobic effect drives a capsular assembly, J. Am. Chem. Soc., 2004, 126, 11408–11409 CrossRef CAS PubMed.
- M. D. Giles, S. Liu, R. L. Emanuel, B. C. Gibb and S. M. Grayson, Dendronized supramolecular nanocapsules: pH independent, water-soluble, deep-cavity cavitands assemble via the hydrophobic effect, J. Am. Chem. Soc., 2008, 130, 14430–14431 CrossRef CAS PubMed.
- R. Kulasekharan and V. Ramamurthy, New water-soluble organic capsules are effective in controlling excited-state processes of guest molecules, Org. Lett., 2011, 13, 5092–5095 CrossRef CAS PubMed.
- H. Gan, C. J. Benjamin and B. C. Gibb, Nonmonotonic assembly of a deep-cavity cavitand, J. Am. Chem. Soc., 2011, 133, 4770–4773 CrossRef CAS PubMed.
- For a review on the complexation studies see: J. H. Jordan and B. C. Gibb, Molecular containers assembled through the hydrophobic effect, Chem. Soc. Rev., 2015, 44, 547–585 RSC and reference cited therein.
- C. L. D. Gibb and B. C. Gibb, Templated assembly of water-soluble nano-capsules: inter-phase sequestration, storage, and separation of hydrocarbon gases, J. Am. Chem. Soc., 2006, 128, 16498–16499 CrossRef CAS PubMed.
- L. S. Kaanumalle, C. L. D. Gibb, B. C. Gibb and V. Ramamurthy, Controlling photochemistry with distinct hydrophobic nanoenvironments, J. Am. Chem. Soc., 2004, 126, 14366–14367 CrossRef CAS PubMed.
- A. Natarajan, L. S. Kaanumalle, S. Jockusch, C. L. D. Gibb, B. C. Gibb, N. J. Turro and V. Ramamurthy, Controlling photoreactions with restricted spaces and weak intermolecular forces: exquisite selectivity during oxidation of olefins by singlet oxygen, J. Am. Chem. Soc., 2007, 129, 4132–4133 CrossRef CAS PubMed.
- A. Greer, Organic chemistry: molecular cross-talk, Nature, 2007, 447, 273–274 CrossRef CAS PubMed.
- L. S. Kaanumalle and V. Ramamurthy, Photodimerization of acenaphthylene within a nanocapsule: excited state lifetime dependent dimer selectivity, Chem. Commun., 2007, 1062–1064 RSC.
- A. K. Sundaresan and V. Ramamurthy, Consequences of controlling free space within a reaction cavity with a remote alkyl group: photochemistry of para-alkyl dibenzyl ketones within an organic capsule in water, Photochem. Photobiol. Sci., 2008, 7, 1555–1564 CAS.
- C. L. D. Gibb, A. K. Sundaresan, V. Ramamurthy and B. C. Gibb, Templation of the excited-state chemistry of alpha-(n-alkyl) dibenzyl ketones: how guest packing within a nanoscale supramolecular capsule influences photochemistry, J. Am. Chem. Soc., 2008, 130, 4069–4080 CrossRef CAS PubMed.
- R. Kulasekharan, M. V. S. N. Maddipatla, A. Parthasarathy and V. Ramamurthy, Role of free space and conformational control on photoproduct selectivity of optically pure α-alkyldeoxybenzoins within a water-soluble organic capsule, J. Org. Chem., 2013, 78, 942–949 CrossRef CAS PubMed.
- A. Parthasarathy and V. Ramamurthy, Role of free space and weak interactions on geometric isomerization of stilbenes held in a molecular container, Photochem. Photobiol. Sci., 2011, 10, 1455–1462 CAS.
- S. R. Samanta, A. Parthasarathy and V. Ramamurthy, Supramolecular control during triplet sensitized geometric isomerization of stilbenes encapsulated in a water soluble organic capsule, Photochem. Photobiol. Sci., 2012, 11, 1652–1660 CAS.
- A. Parthasarathy, S. R. Samantha and V. Ramamurthy, Photodimerization of hydrophobic guests within a water-soluble nanocapsule, Res. Chem. Intermed., 2013, 39, 73–87 CrossRef CAS.
- R. Kulasekharan, R. Choudhury, R. Prabhakar and V. Ramamurthy, Restricted rotation due to the lack of free space within a capsule translates into product selectivity: photochemistry of cyclohexyl phenyl ketones within a water-soluble organic capsule, Chem. Commun., 2011, 47, 2841–2843 RSC.
- S. Gupta, R. Choudhury, D. Krois, G. Wagner, U. H. Brinker and V. Ramamurthy, Photochemical generation and reactivity of carbenes within an organic cavitand and capsule: photochemistry of adamantanediazirines, Org. Lett., 2011, 13, 6074–6077 CrossRef CAS PubMed.
- L. S. Kaanumalle, C. L. D. Gibb, B. C. Gibb and V. Ramamurthy, Photo-Fries reaction in water made selective with a capsule, Org. Biomol. Chem., 2007, 5, 236–238 CAS.
- S. Liu, H. Gan, A. T. Hermann, S. W. Rick and B. C. Gibb, Kinetic resolution of constitutional isomers controlled by selective protection inside a supramolecular nanocapsule, Nat. Chem., 2010, 2, 847–852 CrossRef CAS PubMed.
- A. K. Sundaresan, L. S. Kaanumalle, C. L. D. Gibb, B. G. Gibb and V. Ramamurthy, Chiral photochemistry within a confined space: diastereoselective photorearrangements of a tropolone and a cyclohexadienone included in a synthetic cavitand, Dalton Trans., 2009, 4003–4011 RSC.
- A. K. Sundaresan, C. L. D. Gibb, B. C. Gibb and V. Ramamurthy, Chiral photochemistry in a confined space: torquoselective photoelectrocyclization of pyridones within an achiral hydrophobic capsule, Tetrahedron, 2009, 65, 7277–7288 CrossRef CAS PubMed.
- P. Jagadesan, B. Mondal, A. Parthasarathy, V. J. Rao and V. Ramamurthy, Photochemical reaction containers as energy and electron-transfer agents, Org. Lett., 2013, 15, 1326–1329 CrossRef CAS PubMed.
- V. Ramamurthy and A. Parthasarathy, Chemistry in restricted spaces: select photodimerizations in cages, cavities, and capsules, Isr. J. Chem., 2011, 51, 817–829 CrossRef CAS PubMed.
- N. Jayaraj, P. Jagadesan, S. R. Samanta, J. P. Da Silva and V. Ramamurthy, Release of guests from encapsulated masked hydrophobic precursors by a phototrigger, Org. Lett., 2013, 15, 4374–4377 CrossRef CAS PubMed.
- E. Ramasamy, N. Jayaraj, M. Porel and V. Ramamurthy, Excited state chemistry of capsular assemblies in aqueous solution and on silica surfaces, Langmuir, 2012, 28, 10–16 CrossRef CAS PubMed.
- P. Jagadesan, J. P. Da Silva, R. S. Givens and V. Ramamurthy, Photorelease of incarcerated guests in aqueous solution with phenacyl esters as the trigger, Org. Lett., 2015, 17, 1276–1279 CrossRef CAS PubMed.
- L. R. MacGillivray and J. L. Atwood, A chiral spherical molecular assembly held together by 60 hydrogen bonds, Nature, 1997, 389, 469–472 CrossRef CAS.
- S. Slovak, L. Avram and Y. Cohen, Encapsulated or not encapsulated? Mapping alcohol sites in hexameric capsules of resorcin[4]arenes in solution by diffusion NMR spectroscopy, Angew. Chem., Int. Ed., 2010, 49, 428–431 CrossRef CAS PubMed.
- L. Avram and Y. Cohen, The role of water molecules in a resorcinarene capsule as probed by NMR diffusion measurements, Org. Lett., 2002, 4, 4365–4368 CrossRef CAS PubMed.
- A. Shivanyuk and J. Rebek Jr, Reversible encapsulation by self-assembling resorcinarene subunits, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 7662–7665 CrossRef CAS PubMed.
- A. Shivanyuk and J. Rebek Jr, Reversible encapsulation of multiple, neutral guests in hexameric resorcinarene hosts, Chem. Commun., 2001, 2424–2425 RSC.
- L. C. Palmer, A. Shivanyuk, M. Yamanaka and J. Rebek Jr, Resorcinarene assemblies as synthetic receptors, Chem. Commun., 2005, 857–858 RSC.
- T. Evan-Salem, I. Baruch, L. Avram, Y. Cohen, L. C. Palmer and J. Rebek Jr, Resorcinarenes are hexameric capsules in solution, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12296–12300 CrossRef CAS PubMed.
- L. Avram and Y. Cohen, Discrimination of guests encapsulation in large hexameric molecular capsules in solution: pyrogallol[4]arene versus resorcin[4]arene capsules, J. Am. Chem. Soc., 2003, 125, 16180–16181 CrossRef CAS PubMed.
- S. Slovak and Y. Cohen, In-out interactions of different guests with the hexameric capsule of resorcin[4]arene, Supramol. Chem., 2010, 22, 803–807 CrossRef CAS PubMed.
- I. E. Philip and A. E. Kaifer, Electrochemically driven formation of a molecular capsule around the ferrocenium ion, J. Am. Chem. Soc., 2002, 124, 12678–12679 CrossRef CAS PubMed.
- I. Philip and A. E. Kaifer, Noncovalent encapsulation of cobaltocenium inside resorcinarene molecular capsules, J. Org. Chem., 2005, 70, 1558–1564 CrossRef CAS PubMed.
- G. Bianchini, A. Scarso, G. L. Sorella and G. Strukul, Switching the activity of a photoredox catalyst through reversible encapsulation and release, Chem. Commun., 2012, 12082–12084 RSC.
- L. Avram, Y. Cohen and J. Rebek Jr, Recent advances in hydrogen-bonded hexameric encapsulation complexes, Chem. Commun., 2011, 5368–5375 RSC.
- Q. Zhang and K. Tiefenbacher, Hexameric resorcinarene capsule is a Bronsted acid: investigation and application to synthesis and catalysis, J. Am. Chem. Soc., 2013, 135, 16213–16219 CrossRef CAS PubMed.
- A. Cavarzan, A. Scarso, P. Sgarbossa, G. Strukul and J. N. H. Reek, Supramolecular control on chemo- and regioselectivity via encapsulation of (NHC)-Au catalyst within a hexameric self-assembled host, J. Am. Chem. Soc., 2011, 133, 2848–2851 CrossRef CAS PubMed.
- A. Cavarzan, J. N. H. Reek, F. Trentin, A. Scarso and G. Strukul, Substrate selectivity in the alkyne hydration mediated by NHC-Au(I) controlled by encapsulation of the catalyst within a hydrogen bonded hexameric host, Catal. Sci. Technol., 2013, 3, 2898–2901 CAS.
- S. Giust, G. La Sorella, L. Sperni, G. Strukul and A. Scarso, Substrate selective amide coupling driven by encapsulation of a coupling agent within a self-assembled hexameric capsule, Chem. Commun., 2015, 51, 1658–1661 RSC.
- B. Drake, C. B. Prater, A. L. Weisenhorn, S. A. Gould, T. R. Albrecht, C. F. Quate, D. S. Cannell, H. G. Hansma and P. K. Hansma, Imaging crystals, polymers, and processes in water with the atomic force microscope, Science, 1989, 243, 1586–1589 CAS.
- A. Janshoff, M. Neitzert, Y. Oberdcrfer and H. Fuchs, Force spectroscopy of molecular systems - single molecule spectroscopy of polymers and biomolecules, Angew. Chem., Int. Ed., 2000, 39, 3212–3237 CrossRef CAS.
- F. Kienberger, A. Ebner, H. J. Gruber and P. Hinterdorfer, Molecular recognition imaging and force spectroscopy of single biomolecules, Acc. Chem. Res., 2006, 39, 29–36 CrossRef CAS PubMed.
- D. J. Müller and Y. Dufrêne, Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology, Nat. Nanotechnol., 2008, 3, 261–269 CrossRef PubMed.
- M. Beyer and H. Clausen-Schaumann, Mechanochemistry: the mechanical activation of covalent bonds, Chem. Rev., 2005, 105, 2921–2948 CrossRef CAS PubMed.
- E. U. T. van Velzen, J. F. J. Engbersen, P. J. de Lange, J. W. G. Mahy and D. N. Reinhoudt, Self-assembled monolayers of resorcin[4]arene tetrasulfides on gold, J. Am. Chem. Soc., 1995, 117, 6853–6862 CrossRef.
- H. Schcnherr, G. J. Vancso, B.-H. Huisman, F. C. J. M. van Veggel and D. N. Reinhoudt, Lattice structure of self-assembled monolayers of dialkyl sulfides and calix[4]arene sulfide adsorbates on Au(111) revealed by atomic force microscopy, Langmuir, 1999, 15, 5541–5546 CrossRef.
- P. Hinterdorfer, W. Baumgartner, H. J. Gruber, K. Schilcher and H. Schindler, Detection and localization of individual antibody-antigen recognition events by atomic force microscopy, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 3477–3481 CrossRef CAS.
- T. Schrcder, T. Geisler, B. Schnatwinkel, D. Anselmetti and J. Mattay, Single-molecule force spectroscopy of supramolecular heterodimeric capsules, Phys. Chem. Chem. Phys., 2010, 12, 10981–10987 RSC.
- R. Eckel, R. Ros, B. Decker, J. Mattay and D. Anselmetti, Supramolecular chemistry at the single-molecule level, Angew. Chem., Int. Ed., 2005, 44, 484–488 CrossRef CAS PubMed.
- Y. Gilbert, M. Deghorain, L. Wang, B. Xu, P. D. Pollheimer, H. J. Gruber, J. Errington, B. Hallet, X. Haulot, C. Verbelen, P. Hols and Y. F. Dufrene, Single-molecule force spectroscopy and imaging of the vancomycin/D-Ala-D-Ala interaction, Nano Lett., 2007, 7, 796–801 CrossRef CAS PubMed.
- R. Prasanth, G. Nair and C. M. Girish, Enhanced endocytosis of nano-curcumin in nasopharyngeal cancer cells: An atomic force microscopy study, Appl. Phys. Lett., 2011, 99, 163706 CrossRef PubMed.
- K. J. V. Vliet and P. Hinterdorfer, Probing drug-cell interactions, Nano Today, 2006, 1, 18–25 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |