Design, synthesis and insecticidal activities of N-(4-cyano-1-phenyl-1H-pyrazol-5-yl)-1,3-diphenyl-1H-pyrazole-4-carboxamide derivatives†
Abstract
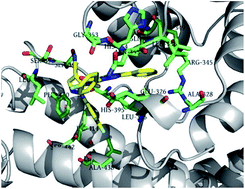
Insect ryanodine receptor is one of the promising targets for the development of novel insecticides. In order to search for potent insecticides targeting the ryanodine receptor (RyR), a series of novel diphenyl-1H-pyrazole derivatives with cyano substituent were designed and synthesized. Their insecticidal activities against diamondback moth (Plutella xylostella) indicated that most of the compounds showed moderate to high activities at the four concentrations. Among these compounds, N-(4-cyano-1-(4-fluorophenyl)-1H-pyrazol-5-yl)-1-(4-fluorophenyl)-3-phenyl-1H-pyrazole-4-carboxamide (5g) showed 84% larvicidal activity against Plutella xylostella at the concentration of 0.1 mg L−1. Molecular docking showed the predicted binding mode between 5g and protein receptor, which could suggest that the title compounds were the possible activators of insect RyR.

 Please wait while we load your content...
Please wait while we load your content...